Well-Being of Child and Family Participants in Phase 1 Pediatric Oncology Clinical Trials
Problem Identification: Pediatric oncology phase 1 clinical trials (P1Ts) are essential to developing new anticancer therapies; however, they raise complex ethical concerns about balancing the need for this research with the well-being of participating children. The purpose of this integrative review was to synthesize and appraise the evidence of how P1T participation, which begins with consent and ends with the transition off the P1T, can affect the well-being (either positively or negatively) of children with cancer. The Resilience in Individuals and Families Affected by Cancer Framework, which has an outcome of well-being, was used to synthesize findings.
Literature Search: Articles on the experiences of child (n = 21) and adult (n = 31) P1T participants were identified through systematic searches.
Data Evaluation: Articles were evaluated on rigor and relevance to P1T participant experiences as high, medium, or low.
Synthesis: Minimal empirical evidence was found regarding the effect of P1T participation on the well-being of children with cancer. Adult P1T participant experiences provide insights that could also be important to children’s P1T experiences.
Implications for Practice: To achieve a balanced approach in P1T consent discussions, nurses and healthcare providers who work with children considering participation in a P1T should share the potential effect of participation on participants’ well-being.
Jump to a section
Cancer remains the leading cause of death by disease in children aged 14 years and younger (Siegel, Miller, & Jemal, 2016). Although five-year survival rates for pediatric cancers have improved overall to 83%, for some pediatric cancers, the five-year survival rate is only 67% (Siegel et al., 2016). New therapies are needed to improve outcomes for children with cancer. Phase 1 clinical trials (P1Ts) are the first step in testing new medical therapies in humans and are essential to the development of innovative therapies for children with cancer (Kim et al., 2008; Lee, Skolnik, & Adamson, 2005).
Although the need for P1Ts is generally accepted, P1Ts raise ethical concerns (Agrawal & Emanuel, 2003; Berg, 2007; Crites & Kodish, 2013; de Vries et al., 2011; Ekert, 2013; Estlin, Cotterill, Pratt, Pearson, & Bernstein, 2000; Hazen, Zyzanski, Baker, Drotar, & Kodish, 2015; Kearns & Morland, 2014; Miller & Joffe, 2008; Oberman & Frader, 2003; Weinfurt et al., 2012). Goals of P1Ts are traditionally to determine the maximum-tolerated dose of the therapy, describe the action of the therapy in humans, and reveal side effects (Kim et al., 2008; Lee et al., 2005). The National Cancer Institute updated P1T templates to include preliminary determination of efficacy as a secondary trial objective (Weber et al., 2015). P1Ts are primarily conducted to determine how innovative therapies may safely be given. Although not intended to directly benefit participants, healthcare providers and researchers hope that P1Ts will directly benefit at least some participants. The Declaration of Helsinki requires that “while the primary purpose of medical research is to generate new knowledge, this goal can never take precedence over the rights and interests of individual research subjects” (World Medical Association, 2013, p. 2). The ethical challenge of P1Ts is to ensure that the well-being of participants is supported throughout the trial.
Ethical concerns regarding P1Ts are more complex in children with cancer, in part because children are only eligible for P1Ts when no known curative therapy remains for their cancer. In addition, children must rely on their parents as proxy decision makers (Ekert, 2013; Estlin et al., 2000; Kearns & Morland, 2014; Oberman & Frader, 2003). Enrolling a child in a P1T provides the child access to a novel investigational therapy of promising, albeit uncertain, efficacy (Bluebond-Langner, Belasco, Goldman, & Belasco, 2007; Mack et al., 2008; Tomlinson et al., 2011). Parents enroll their children in P1Ts primarily based on hope of a cure for their child from the novel therapy (Barrera, D’Agostino, Gammon, Spencer, & Baruchel, 2005; Crites & Kodish, 2013; Deatrick, Angst, & Moore, 2002; Oppenheim, Geoerger, & Hartmann, 2005). However, the median life expectancy of children after enrollment in pediatric oncology P1Ts is 3.6–6.4 months (Bautista et al., 2015; Kim et al., 2008; Morgenstern et al., 2014). Consequently, children with cancer enrolled in P1Ts spend part of what could be limited time remaining being treated in a P1T that is not focused on directly benefiting them, although healthcare providers and researchers hope that they will indeed benefit. Ethicists and healthcare providers identify some potential benefits of pediatric oncology P1T participation as improved quality of life (QOL) and hope, although the risks include fostering unrealistic hope, burdening children with additional medical procedures and toxicities, and limiting the opportunity for palliation (Barnes, Pressey, Adams, Hensler, & Madan-Swain, 2014; Beardsmore & Fitzmaurice, 2002; Carlson, Reilly, & Hitchens, 2005; Chang, 2008; Estlin et al., 2000; Gilliam, Madan-Swain, Adams, & Pressey, 2013; Oberman & Frader, 2003; Ulrich, Grady, & Wendler, 2004). To avoid inadvertently negatively affecting the well-being of children with cancer near the end of life and to provide evidence to inform future pediatric oncology P1T participants, a better understanding of how P1T participation affects the well-being of children with cancer is needed.
The purpose of this integrative review was to synthesize and appraise the evidence of how P1T participation can positively or negatively affect the well-being of children with cancer. P1T participation is defined as beginning at the start of the informed consent process, extending through enrollment and receiving therapy, and ending at transition off the P1T. Because of the substantially greater number of P1Ts for adults with cancer, the similar structure and requirements of P1Ts in the pediatric and adult populations, the dearth of pediatric literature, and the possibility of gaining insights from adults’ P1T experiences, this review includes pediatric and adult literature searches.
Resilience in Individuals and Families Affected by Cancer Framework
Review findings were synthesized using the Resilience in Individuals and Families Affected by Cancer Framework (Resilience Framework), the organizing framework for nursing research conducted through the Children’s Oncology Group (COG) (Kelly, Hooke, Ruccione, Lanier, & Haase, 2014; Landier, Leonard, & Ruccione, 2013). The Resilience Framework is used to facilitate an understanding of how children and families sustain or regain well-being after a pediatric cancer diagnosis, and to guide interventions that can promote child and family well-being (Haase et al., 2017; Haase, Kintner, Monahan, & Robb, 2014; Kelly et al., 2014; Landier et al., 2013) (see Figure 1). The framework was developed from Haase’s Resilience in Illness model, which was empirically derived from research with pre-adolescents and adolescents with cancer (Haase et al., 2014, 2017; Haase, Heiney, Ruccione, & Stutzer, 1999). For children participating in P1Ts, the Resilience Framework can provide an organizing structure for understanding how P1T experiences affect the children’s overall well-being. 
The Resilience Framework includes two risk factors (illness-related distress and defensive coping); four protective factors (courageous coping, social integration, family environment, and derived meaning), and one outcome factor (well-being) (Kelly et al., 2014; Landier et al., 2013). The risk factors are negatively associated with well-being, and the protective factors are positively associated with well-being (see Figure 2). The well-being outcome factor includes positive health outcomes of global QOL, resilience, a sense of confidence and mastery, and self-transcendence (Kelly et al., 2014). Social integration includes the support of family, friends, healthcare providers, and the community (Haase et al., 2014, 2017; Kelly et al., 2014). 
To ensure burdens associated with P1T participation were captured, treatment burden was an additional indicator of the illness-related distress risk factor. Treatment burden refers to the physical, financial, time, psychosocial, and procedural demands that a treatment places on a patient and/or the family (Eton et al., 2012; Sav, Kendall, et al., 2013; Sav, King, et al., 2013; Ziaian et al., 2006).
According to the Resilience Framework, courageous and defensive coping are not mutually exclusive; some coping strategies encompass elements of both factors (Kelly et al., 2014). Work undertaken in the development of the Resilience Framework (i.e., Haase’s Resilience in Illness model) confirms that defensive coping strategies are normal responses to high-threat situations that are used until courageous coping strategies are developed to address the threat (Haase et al., 2017). Defensive coping strategies are problematic only when used exclusively and for prolonged periods of time, without development of courageous coping strategies (Haase et al., 2017).
Literature Search
This integrative review was conducted using the Whittemore and Knafl (2005) method. This method guides each stage of the review (i.e., problem identification, literature search, data evaluation, data analysis, and presentation of findings). Two literature searches were performed in December 2016 in PubMed and CINAHL Complete (EBSCO) databases. The first search focused on pediatric literature, whereas the second focused on adult literature.
The first search used the keywords (a) cancer or oncology; (b) child or pediatric; and (c) phase 1; phase I; clinical trials; phase I (as topic); therapies, investigational; early phase; or early trials. In total, 2,386 articles were identified. For this review, children were defined as individuals aged younger than 18 years. Excluded criteria were expert opinions and/or theoretical discussions, non-English, published prior to 1985, lacking an abstract, or not relevant to the purpose of the review. The date range was selected to obtain a large sample focused on more recent pediatric oncology P1Ts, which tend to use more molecularly targeted therapies and involve more efficient trial designs. Articles concentrating solely on clinical trial procedures (e.g., the quality of the P1T consent process) rather than the experiences of participants were excluded. The reference lists of the included articles were examined using the search criteria, resulting in a total of 21 remaining articles (see Figure 3). Only one of these articles was highly relevant to the purpose of this review. 
The second search focused on adult P1T participants and was conducted after the pediatric search was completed. Although the findings had to be interpreted cautiously, the authors felt that this search was particularly important given the dearth of highly relevant articles in the pediatric literature. Keywords were similar to previous keywords, but excluding child and pediatric. Because 17,197 mainly irrelevant articles were identified, the following keywords were used to refine the adult literature search: participation, qualitative research, psychosocial factors, burden, or supportive. In total, 3,237 articles were identified and screened. In addition, because informed consent procedures are fundamentally different with adult and pediatric participants, articles that solely considered the P1T consent experience were excluded. After screening and reviewing the reference lists, 31 empirical articles that described the experience or well-being of adults during their participation in oncology P1Ts were identified (see Figure 4). 
Data Evaluation
According to the Whittemore and Knafl (2005) method, all articles were evaluated by the authors based on relevance to the review’s purpose and empirical rigor (i.e., the description of the research methods). Scores were separately ranked high, medium, or low for relevance and rigor. The first author assessed all articles in the sample, and the second author independently performed a confirmatory assessment of 10 randomly selected articles (five pediatric and five adult). Articles were not excluded based on their data evaluation ratings; however, the ratings were considered during analysis when determining the level of available supporting evidence (Whittemore & Knafl, 2005). Table 1 summarizes the article evaluation results. 
Findings From the Pediatric Literature
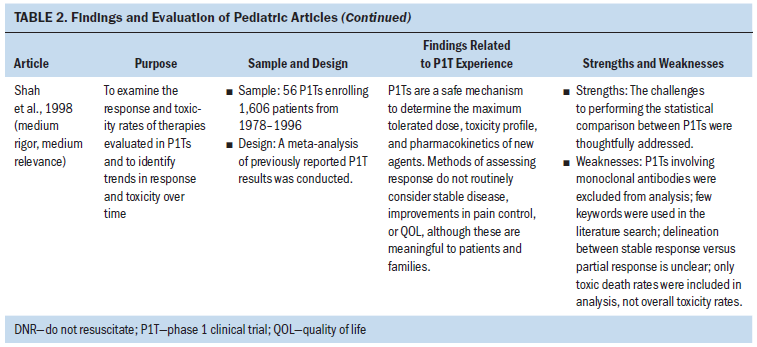
Of the 21 pediatric articles, 12 were empirical research studies, 1 was a case report, and 8 were meta-analyses of results from multiple P1Ts. Of the 12 empirical studies, 2 analyzed the end-of-life care provided to P1T participants, 1 considered nurses’ perceptions of P1Ts, and the remaining 9 examined experiences during the P1T consent processes. With the exception of P1T consent processes, minimal empirical evidence regarding the experiences or well-being of children with cancer during their P1T participation was found. In particular, no studies directly examined the experiences of children with cancer throughout a P1T. Findings from the pediatric literature are synthesized and presented by the factors of the Resilience Framework (see Table 2). 


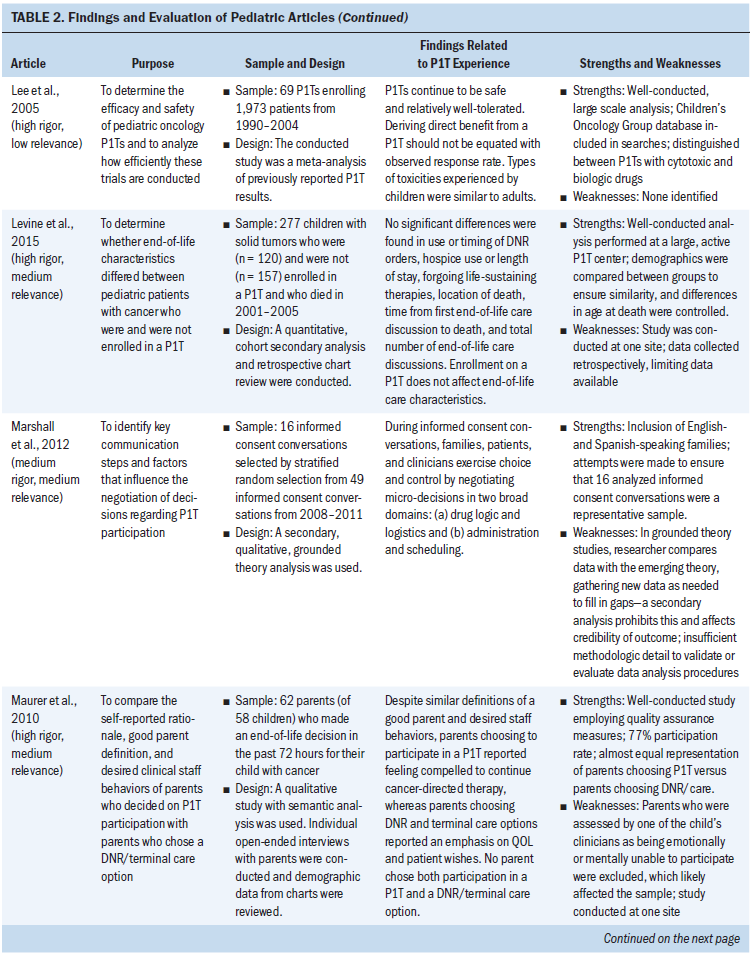

Risk Factor: Illness-Related Distress
For children with cancer and their parents considering participation in a P1T, some evidence supported the association of that overall well-being with being able to perform usual physical activities, as well as not experiencing side effects from cancer or its treatments (Barrera et al., 2005). Performance scores are a measure of general well-being and ability to complete activities of daily living (Lansky, List, Lansky, Ritter-Sterr, & Miller, 1987; Mor, Laliberte, Morris, & Wiemann, 1984). Because P1Ts have performance status eligibility requirements, as expected, at enrollment, children had high performance scores overall, despite being heavily pretreated for their cancer (Kim et al., 2008).
Regarding outcomes of P1T participation, the pediatric meta-analyses established that, for most children, their cancer did not improve. The combined partial and complete response rate for children enrolled in P1Ts was 3.8%–9.6%, with reports of 17%–24.5% prolonged disease stabilization (i.e., more than three or four months) (Bautista et al., 2015; Furman et al., 1989; Kim et al., 2008; Lee et al., 2005; Morgenstern et al., 2014; Shah et al., 1998). Although the median overall survival time of children with relapsed cancer after enrollment in a P1T was 3.6–6.4 months, Morgenstern et al. (2014) reported 16% of children survived longer than 12 months, and Kim et al. (2008) reported 5% survived longer than 36 months. The median time in a P1T was 1.3–1.8 months, or one cycle (Bautista et al., 2015; Kim et al., 2008; Morgenstern et al., 2014). Overall, 13%–24% of children with cancer experienced a dose-limiting toxicity, 46.7% experienced a grade 3 or 4 toxicity, 7.6% were hospitalized because of toxicities, and 0%–2.4% of children died related to toxicities experienced during a P1T (Bautista et al., 2015; Furman et al., 1989; Kim et al., 2008; Lee et al., 2005; Morgenstern et al., 2014; Shah et al., 1998). Although overall death rates of children participating in P1Ts ranged from 7%–21%, progressive disease accounted for most of the deaths during P1Ts (Furman et al., 1989; Shah et al., 1998). Despite being more heavily pretreated, children had a similar or greater medication tolerance than adults enrolled on matched P1Ts (Carlson, Ho, Smith, Reisch, & Weitman, 1996; Paoletti et al., 2013). No other symptom experience data were found for pediatric P1T participants.
Illness-related uncertainty was not well explored in pediatric P1Ts. Parents considering having their child participate in a P1T spoke of high uncertainty related to unknowns and the extra testing needed to verify eligibility (Barrera et al., 2005). Although empirical research was not found on children’s experiences during P1Ts, there was some evidence that P1T logistics and potential impact on QOL contributed to parents’ and children’s P1T decision making (Deatrick et al., 2002; Marshall et al., 2012; Maurer et al., 2010; Miller et al., 2013).
Risk Factor: Defensive Coping
No data were found regarding how P1T participation affected or related to the use of defensive coping strategies. Experts hypothesized that participation in a pediatric oncology P1T exacerbates the reluctance of families and healthcare providers to address end-of-life issues in children with cancer (Beardsmore & Fitzmaurice, 2002; Brock, Steineck, & Twist, 2016; Chang, 2008).
Protective Factor: Courageous Coping
Some courageous coping strategies, including maintaining normalcy and control over daily life, spending time with family, and focusing on QOL, were important to children with cancer and their parents considering participation in a P1T (Barrera et al., 2005; Hinds et al., 2005). No other specific data were found on how courageous coping strategies were used by children participating in P1Ts.
Interaction of Defensive and Courageous Coping
Although the optimism associated with clinical trial participation was potentially beneficial for P1T participants (a courageous coping strategy), potential harm could be created by unrealistic optimism (a defensive coping strategy) (Chang, 2008; Deatrick et al., 2002; Helft, Hlubocky, Wen, & Daugherty, 2003; Kearns & Morland, 2014; Meyers et al., 2004). Unrealistic optimism is the belief, regardless of the quality and clarity of information communicated regarding a P1T, that one has a greater chance of personal benefit from P1T participation than any other patient (Crites & Kodish, 2013; Jansen et al., 2016). All P1T participants (or their parents/guardians) sign an informed consent document acknowledging their understanding of the nature of the P1T. Despite this, there was substantial evidence that pediatric P1T participants and families thought and acted like the P1T therapy would improve, or even cure, their cancer (Cousino et al., 2012; Deatrick et al., 2002; Hinds et al., 2005; Maurer et al., 2010; Miller et al., 2013).
In two studies conducted in medical centers with active palliative care programs, mixed evidence was seen regarding the effect of P1T participation and coping on end-of-life care. One study found that P1T participants more frequently enrolled in hospice and were more likely to die at home or in hospice (Brock et al., 2016). The second study found that the end-of-life care provided to pediatric P1T participants did not differ from non–P1T participants (Levine et al., 2015).
Protective Factor: Social Integration
Although the pediatric articles generally reinforced the importance of strong social support to the patient’s well-being, the lack of pediatric empirical research prohibited an understanding of how P1T participation affected children’s social integration. However, regarding healthcare providers’ role in social integration, strong evidence suggested that pediatric P1T participants were included in decision-making discussions with providers (Cousino et al., 2012; Miller et al., 2013).
Protective Factor: Family Environment
Family environment was generally important to P1T participants’ well-being; however, minimal empiric data exist on the role and the impact of family during the P1T experience. Double protection was identified in the pediatric articles, reflecting a potential threat to the protection offered by the family environment. Double protection occurs when both parent and child avoid open communication in an attempt to protect each other from distress (Last, 1992). Although based on an intention to be supportive, double protection results, instead, in isolation and lack of support (Last, 1992). Barrera et al. (2005) and Hinds et al. (2005) provided empirical evidence that children recently enrolled in a P1T were aware of their advanced cancer and of their parents’ emotional turmoil. This suggests that the child’s P1T assent may be influenced by a desire to ease their parents’ suffering or to acquiesce to their parents’ wishes (Barrera et al., 2005; Hinds et al., 2005).
Protective Factor: Derived Meaning
Some evidence suggested that, for children with cancer, P1T participation offered hope that positively influenced well-being (Barrera et al., 2005; Carlson, Bultz, & Morris, 2005). However, parents considering enrolling their child in a P1T struggled to balance hope for a cure with the potential negative effect of the P1T on their child’s well-being (Barrera et al., 2005). Parents who were offered the option of a P1T for their child with cancer held on to different forms of hope, including hope for a peaceful death (Barrera et al., 2005).
Outcome Factor: Well-Being
Although minimal empirical evidence exists regarding how well-being (i.e., QOL) can be affected by P1T participation, there was a high level of evidence that well-being was a contributing factor to pediatric oncology P1T decision-making (Barrera et al., 2005; Deatrick et al., 2002; Hinds et al., 1997, 2005, 2009; Maurer et al., 2010; Miller et al., 2013).
Findings From the Adult Literature
All 31 adult-focused articles were empirical articles exploring adult patient experiences during a P1T. Findings from the adult literature are used to provide insights into children’s experiences in P1Ts and are also synthesized and presented per the factors of the Resilience Framework (see Table 3). 
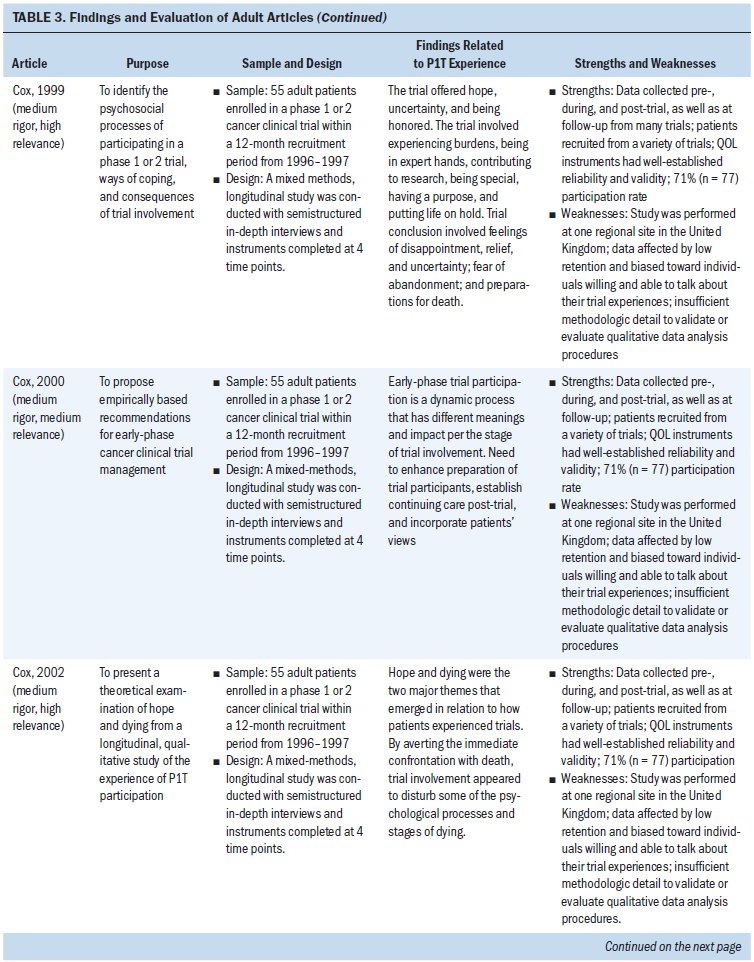







Risk Factor: Illness-Related Distress
Similar to children, adult P1T participants overall had high performance scores at enrollment (Berdel et al., 1988; Finlay, Lu, Henderson, O’Dwyer, & Casarett, 2009; Rouanne et al., 2013). No significant changes in performance or overall QOL scores were found in adult participants who completed one course of P1T therapy (Berdel et al., 1988; Cox, 2003; Melink, Clark, & Von Hoff, 1992), although Rouanne et al. (2013) found that the physical health of adult participants significantly decreased during one course of P1T therapy.
In terms of distress related to symptoms of disease, adult P1T participants had similar or higher levels of symptom burden than nonparticipants (Finlay et al., 2009; Hui et al., 2010). However, George et al. (2016) found that adult P1T participants experienced poor sleep quality, which was connected to increased symptom burden and disturbances in temperament. Some limited evidence suggested that being in a P1T may have positively influenced adults’ experience of their symptoms by providing hope for therapeutic benefit (Cox, 2003; Kvale, Woodby, & Williams, 2010). Of note, Rouanne et al. (2013) found that the level of severe depression in adult P1T participants (2%) was strikingly lower than in the general cancer population (10%–25%) and the general healthy population (5%). There was a high level of evidence for incorporating palliative care and/or hospice simultaneously with adult P1T participation to enhance supportive care and symptom management and to decrease psychological distress (Agrawal & Danis, 2002; Bluebond-Langner et al., 2007; Casarett, Karlawish, Henry, & Hirschman, 2002; Cassel et al., 2016; Hui et al., 2010; Kapo & Casarett, 2002; Meyers et al., 2004; Sun et al., 2014).
For adults, P1T treatment burdens significantly affected their overall QOL (Cohen et al., 2007; Mack, 1998). These burdens included frequent hospital visits, additional medical procedures and tests, toxicities related to the novel therapy, logistical problems such as transportation and parking, and financial cost (Cohen et al., 2007; Cox, 1999; Cox & Avis, 1996; Mack, 1998). Although these burdens were manageable and became routine for some participants, others continuously struggled with them and feared they would become more than they could handle (Cox, 1999). A strong level of evidence suggested that, before entering the P1T, adult participants were either unaware of or underestimated the practical burdens of being in the trial (Cohen et al., 2007; Cox, 1999). After enrolling in the P1T, these burdens became more significant. Taking part in a P1T was compared to having a job in that there was a strict schedule to follow and specific tasks to be performed (Cohen et al., 2007; Cox, 1999; Cox & Avis, 1996; Mack, 1998; Wootten, Abbott, Siddons, Rosenthal, & Costello, 2011). Overall, adults participating in a P1T experienced a sense of life being on hold during the P1T; it was difficult to plan for activities because their lives revolved around the trial and its requirements (Cox, 1999; Cox & Avis, 1996; Stetz, 1993; Wootten et al., 2011).
The role of illness-related uncertainty (which includes complexity and ambiguity) was not well explored in the P1T experience of adults (Haase et al., 2014; Kelly et al., 2014). For adults, there may be more uncertainty experienced during P1T participation than during standard treatments related to the investigational nature of the novel therapy (Cox, 1999; Wootten et al., 2011). However, some evidence suggested that, for adult participants, the specific requirements involved with the P1T may have reduced uncertainty by providing a set plan to follow (Cox & Avis, 1996; Mack, 1998; Moore, 2001; Wilson, Cox, & Elkan, 2005).
In addition, although there are many unknowns regarding the P1T therapy and its potential effect on humans, the information that was available from preclinical and early testing helped P1T participants cope with the uncertainty (Cox & Avis, 1996; Mack, 1998; Wilson et al., 2005).
Risk Factor: Defensive Coping
Some evidence suggested that adult P1T participants employed evasive coping strategies. Adult P1T participants who avoided transitioning to end-of-life care demonstrated the strongest evidence of evasive coping (Cox, 2002; Cox & Avis, 1996; Meyers et al., 2004). Some evidence also suggested that adult P1T participants were at risk for using evasive coping strategies by over-relying on healthcare providers and becoming passive recipients of care (Cox, 2000). This over-reliance was demonstrated by 80% of adult P1T participants who wanted the healthcare provider to tell them what they should do during trial recruitment (Cox, 2000).
Protective Factor: Courageous Coping
Overall, there was a high level of evidence that participating in a P1T was generally a positive experience that supported courageous coping and optimism in adults. This was related to participants’ sense of receiving additional treatment, trying something new, having the support of expert medical care, engaging with the healthcare team, contributing to cancer research, and/or having a purpose in their lives (Campbell & Whyte, 1999; Cox, 1999; Cox & Avis, 1996; Godskesen, Nygren, Nordin, Hansson, & Kihlbom, 2013; Hutchison, 1998; Moore, 2001; Wootten et al., 2011). The stories of adults participating in a P1T consistently reflected courageous coping in attempting the unknown as well as in managing any resulting consequences (Cox & Avis, 1996; Moore, 2001; Stetz, 1993). For many adult patients, P1T participation fulfilled their need to have tried everything to fight their cancer (Cox, 2002; Cox & Avis, 1996; Moore, 2001; Stetz, 1993).
Interaction of Defensive and Courageous Coping Factors
Adult P1T participants, similar to pediatric participants, expressed an expectation that the P1T therapy would improve, or even cure, their cancer (Godskesen et al., 2013; Helft et al., 2003; Jansen et al., 2016). However, expectations for tumor response and symptom improvement were not generally met during P1Ts (Campbell & Whyte, 1999; Mack, 1998; Wootten et al., 2011; Yoder, O’Rourke, Etnyre, Spears, & Brown, 1997). How significant a problem unrealistic optimism was for adult P1T participants was unclear (Helft et al., 2003; Jansen et al., 2016; Weinfurt et al., 2012). Some evidence suggested that adult P1T participants were able to be realistic about their prognosis and still hopeful about the P1T, which supports Weisman’s theory that patients may simultaneously accept and deny the dying experience (Carlson, Bultz, & Morris, 2005; Daugherty et al., 2005; Godskesen et al., 2013; Moore, 2001; Stetz, 1993; Weisman, 1972).
Some evidence also suggested defensive and courageous coping strategies being used by adult P1T participants at trial conclusion. Trial conclusion was a particularly difficult time for adult P1T participants because it frequently signaled cancer progression and was accompanied by a loss of optimism (Cox, 1999, 2002; Cox & Avis, 1996; Moore, 2001; Wilson et al., 2005; Wootten et al., 2011). Despite this, there was evidence that adult P1T participants at the conclusion of the trial maintained hope that others would be helped by their participation in the P1T (Cox, 2002; Wilson et al., 2005; Wootten et al., 2011). At the conclusion of the trial, adult P1T participants also coped with disappointment that the P1T did not work for them, relief that they no longer had to manage the P1T burdens, a loss of control when the decision to leave the P1T was made for them by healthcare providers/researchers, and fear of abandonment by the medical experts that had been caring for them (Cox, 1999; Cox & Avis, 1996; Moore, 2001; Wilson et al., 2005; Wootten et al., 2011).
The articles describing adults’ P1T experiences included discussion regarding whether P1T participation represented survival work that detracted from death work (Cox, 2002; Meyers et al., 2004; Moore, 2001). Survival work refers to the cognitive and behavioral tasks involved with choosing to seek further treatment to improve and/or cure one’s disease, versus letting the disease take its natural course (Stetz, 1993). Death work consists of the tasks involved with preparing for one’s death practically, emotionally, socially, and spiritually (Schou, 1992). Because most P1T participants died during or shortly after trial conclusion, many adult P1T participants engaged in significant survival work and lost opportunities to complete death work (Campbell & Whyte, 1999; Cox, 2002; Stetz, 1993). This trade-off in survival and death work was another example of the interaction between courageous and defensive coping strategies: For P1T participants, survival work reflected courageous coping, whereas the simultaneous avoidance of death work reflected defensive coping.
The idea that death work may be altered by P1T participation was grounded in Glaser and Strauss’s (1965, 1968) findings that dying involves a psychological process of adjustment, and the most crucial phase in the dying process occurs with recognition that a cure is not possible and that death will occur in the foreseeable future. However, instead of viewing P1T participation as lost time where death work was not completed, P1T participation may better be viewed as one of the different trajectories that can be taken in the dying process (Cox, 2002; Moore, 2001). There was no evidence that adult P1T participants would have engaged in death work had they not enrolled in a P1T (Cox, 2002; Moore, 2001).
Protective Factor: Social Integration
Overall, the adult articles reinforced the importance of strong social support. There was limited evidence that adult P1T participants reported higher levels of social integration than nonparticipants (Berdel et al., 1988).
Cox described the therapeutic alliance formed between adult P1T participants and healthcare providers, where P1T participants benefited from the sense of the alliance working together to actively fight their cancer (Cox, 1999; Moore, 2001). There was strong evidence that adult P1T participants experienced enhanced support of high-quality healthcare and valued effective communication with healthcare providers (Cox, 1999; Cox & Avis, 1996; Hutchison, 1998; Moore, 2001; Wootten et al., 2011; Yoder et al., 1997). However, adult P1T participants who traveled to a different location to participate in the P1T were adversely affected by losing the support of their original healthcare providers (Cohen et al., 2007). Adult P1T participants also experienced feelings of abandonment if their connection and shared goals with the P1T healthcare providers was lost at the end of trial participation (Cox, 1999; Cox & Avis, 1996; Wilson et al., 2005). Adult P1T participants frequently experienced gaps in support and information at trial conclusion and would have benefited from assistance coping by being removed from the trial and transitioning back to their original healthcare providers (Cox, 1999; Cox, Wilson, Arthur, Elkan, & Armstrong, 2005; Wilson et al., 2005; Wootten et al., 2011; Yoder et al., 1997).
An important societal influence prevalent in Western culture is the expectation that people with cancer are to be brave and fight to overcome their cancer (Moore, 2001). There was some limited evidence that this societal expectation could influence adults’ participation in a P1T, because stopping treatment could be viewed as giving in and losing hope (Moore, 2001; Wootten et al., 2011). Societal influences on P1T participation were not explored in the pediatric articles.
Protective Factor: Family Environment
Family environment was generally important to adult P1T participants’ well-being (Campbell & Whyte, 1999; Ulrich et al., 2012; Wootten et al., 2011; Yoder et al., 1997). In particular, the inclusion of the adult participant’s family regarding the P1T plan of care enhanced communication (Wootten et al., 2011; Yoder et al., 1997). Adult participants expected increased support from their family during the P1T and tended to receive support beyond those expectations (Yoder et al., 1997). There was also some evidence that traveling away from family to participate in a P1T adversely affected the well-being of adult P1T participants (Cohen et al., 2007). Kessler et al. (2014) found that caregivers of adult P1T participants reported high levels of distress, anxiety, and depressive symptoms, suggesting that enrollment in a P1T places a considerable burden on family and caregivers in terms of scheduling and managing the patient’s care.
Protective Factor: Derived Meaning
There was strong evidence that P1T participation offered hope that positively influenced well-being in adults (Cox, 1999, 2002; Cox & Avis, 1996; Godskesen et al., 2013; Moore, 2001; Stetz, 1993; Ulrich et al., 2012; Wootten et al., 2011). Adult P1T participants attributed meaning to simply being offered the opportunity to participate in a P1T; the offer engendered feelings of being special and chosen because only a few individuals were extended the opportunity (Cox, 1999, 2000, 2002; Godskesen et al., 2013; Stetz, 1993).
When prematurely removed from a P1T because of disease progression or toxicities, adult P1T participants lost hope for stabilization of cancer and/or symptom improvement (Cox, 2002; Wilson et al., 2005; Wootten et al., 2011). Despite losing hope for self, there was also evidence that participants maintained hope that others would be helped by their P1T participation (Barrera et al., 2005; Cox, 2002; Wilson et al., 2005; Wootten et al., 2011). Even if they did not personally benefit, adult P1T participants generally did not regret participating in the trial. Having the opportunity to try a novel treatment, helping themselves and others, and contributing to future scientific advances all provided meaning for adults’ P1T experiences (Carlson, Bultz, & Morris, 2005; Cox, 1999; Godskesen et al., 2013; Mack, 1998; Ulrich et al., 2012; Wootten et al., 2011; Yoder et al., 1997).
Outcome Factor: Well-Being
QOL was the only well-being outcome assessed in the articles (Kelly et al., 2014). For adult P1T participants, QOL was focused on the ability to function, be productive, and be free from symptoms of disease and treatment side effects (Campbell & Whyte, 1999; Cohen et al., 2007; Wootten et al., 2011). In adult participants, no significant changes were found in overall QOL scores at the end of one course of P1T therapy (Berdel et al., 1988; Cox, 2003; Melink et al., 1992; Rouanne et al., 2013). Some evidence suggested that older adults participating in P1Ts evaluated their QOL in comparison with their own prior treatment experiences and health, as well as in comparison with peers (Cohen et al., 2007; Kvale et al., 2010).
Discussion
Overall, this review established that minimal empirical evidence exists in the pediatric literature regarding the experiences or well-being of children with cancer in a P1T, beyond the process of consenting to the P1T. This lack of pediatric-focused research restricts the understanding of the effect of pediatric oncology P1T participation on the well-being of children. Findings from the adult literature provide insights into adults’ P1T experiences that could be similar for child participants and suggest areas for consideration in future research. Table 4 compares the findings from the pediatric and adult literature for each factor of the Resilience Framework. 

The highest level of evidence in the pediatric literature is from the meta-analyses of P1T outcomes (Bautista et al., 2015; Carlson et al., 1996; Furman et al., 1989; Kim et al., 2008; Lee et al., 2005; Morgenstern et al., 2014; Paoletti et al., 2013; Shah et al., 1998). Although the prognosis for children with cancer enrolled in a P1T is poor, pediatric oncology P1Ts historically are safe, have manageable toxicities, and offer the potential for at least stabilization of disease for several months. Findings from the adult literature that appear to be relevant to children participating in a P1T include the following: P1T-related treatment burdens may include increased hospital visits, additional medical procedures and tests, toxicities related to the novel therapy, logistical problems such as transportation and parking, and financial cost. The role of strong family and social support in fostering P1T participants’ well-being was well-recognized. It is important to also recognize the burden that P1T participation places on family members, and that travel away from family and friends to participate in a P1T may adversely affect the well-being of P1T participants.
One idea from the adult literature that could be important for child participants is that, because P1T participation focuses providers’, patients’, and families’ attention on survival work (courageous coping), P1T participation may foster avoidance of death work (defensive coping) that could negatively affect the patient’s dying process and/or the family’s bereavement. However, it is unclear whether adult P1T participants would have chosen to engage in death work had they not participated in a P1T. In addition, defensive coping strategies only become problematic when used exclusively for long periods of time, without development of courageous coping strategies (Haase et al., 2017). In fact, the adult literature findings include that P1T participation supported courageous coping strategies by providing a sense of trying everything, allowing participants to form a therapeutic alliance with healthcare providers, representing a contribution to cancer research, and providing a purpose and meaning for their lives.
In the pediatric literature, the effect of P1T participation on death work was only minimally studied. Some evidence suggested that parents of children with cancer were only able to consider non-curative options, such as hospice, after accepting that their child could not get better (Hinds et al., 1997; Maurer et al., 2010). Ethicists and healthcare providers hypothesized that the participation of children with cancer in P1Ts not only alters their own death work, but also the grief work of their parents (who serve as proxy decision makers) (Meyers et al., 2004; Oberman & Frader, 2003). In particular, Meyers et al. (2004) proposed that a child’s P1T participation could theoretically minimize parents’ ability to engage in anticipatory grieving, potentially leading to more incidences of complicated bereavement. However, no empirical evidence exists that supports this theory.
A second idea from the adult literature that could be important for child participants is that external influences could affect the decision to participate in a P1T. Although not explored in the pediatric articles, additional research is warranted regarding whether children might feel compelled to battle their cancer heroically, and to live up to societal—and perhaps familial—expectations. The unnaturalness of death in children and the fact that multiple stakeholders (i.e., child, parents, and providers) need to come to an agreement to stop cancer-focused therapy are potential influencers on the decision to participate in a pediatric oncology P1T. Double protection was briefly explored in two of the pediatric articles. Because of the vital importance of the parent–child relationship for children, double protection may also be particularly influential in children’s assent to participate in a P1T as they seek to ease their parents’ suffering or to acquiesce to their parents’ wishes (Broome & Richards, 2003).
Limitations
Literature searches were limited to articles in the English language and with an abstract. The experiences of adults are likely different in some important ways than the experiences of children, requiring that the conclusions derived from the adult literature be interpreted with caution. However, the dearth of articles focused on the experiences of children necessitated this approach and highlights the need for future research focused on children’s experiences. In addition, many of the articles in the sample were published more than 10 years ago and would not have reflected recent advances in molecularly targeted therapies and/or trial designs. Lastly, article evaluations were susceptible to bias because the authors, journal, and publication year were not blinded to researchers.
Implications for Nursing Practice and Research
Review findings have implications for nurses who work with children and families considering participation in a pediatric oncology P1T. First, the findings provide reassurance that, historically, P1Ts are generally safe and may indeed provide direct benefit for children with cancer. Second, the idea that a child’s assent to participate in a P1T may not solely reflect his or her own desires, but instead may be influenced by a desire to ease the parents’ suffering or by family and societal expectations, warrants nurses’ attention. Children should have an active voice in P1T decision making and be directly addressed in consent discussions at an appropriate developmental level (Baker et al., 2013; Broome & Richards, 2003; Miller, Baker, Leek, Drotar, & Kodish, 2014). Third, communication during pediatric oncology P1T conversations is often falsely reassuring (Miller, Cousino, Leek, & Kodish, 2014). Including the potential impact of P1T participation on children’s well-being, per the findings of this review, will help to achieve a balanced approach when presenting the risks and potential benefits of participation during P1T consent discussions and will help to ensure that pediatric oncology P1T participants are well-informed when making decisions about P1T enrollment.
The methods used to research the experience of adult P1T participants may be helpful to guide future research with pediatric participants. In particular, the work of nurse scientist Karen Cox, PhD, is noteworthy in that it provides an exemplar of how research into adult experiences in P1Ts developed from initial descriptive studies into testing of an intervention to improve the transition off of a P1T (Cox, 1998, 1999, 2000, 2002, 2003; Cox & Avis, 1996; Cox et al., 2005). 
Conclusion
Although research on how P1T participation may affect the well-being of children with cancer is limited, the adult literature provides insight into adults’ P1T experiences that could be similar for child participants and suggests areas for consideration in future research. The experience and effects of P1T participation on children’s well-being needs to be understood to ensure that the ethical principles outlined in the Declaration of Helsinki are upheld (World Medical Association, 2013). Understanding the effect of P1T participation on participants’ well-being provides nurses and other healthcare providers with the opportunity to incorporate participants’ experiences into the management of P1Ts and the preparedness of future participants (Cox, 2000). Future research needs to focus on achieving a better understanding of the effect of P1T participation on children’s well-being to address significant gaps in the understanding of this experience and to ensure that P1T participation does not inadvertently negatively affect the well-being of children with cancer.
About the Author(s)
Stacey Crane, PhD, RN, CPON®, is a postdoctoral fellow, Joan E. Haase, PhD, RN, FAAN, is a Holmquist Professor of Pediatric Oncology Nursing, and Susan E. Hickman, PhD, is a professor, all in the School of Nursing at Indiana University in Indianapolis. This research was funded by grants from the National Institute of Nursing Research (F31 NR015393 and T32 NR007066), the National Cancer Institute (T32 CA117865), and the American Cancer Society (DSCN-13-267-01-SCN and DSCNR-15-081-03-SCN); the 2016 ONS Foundation Dissertation Research Grant; and a Midwest Nursing Research Society Founder’s Circle endowment grant. All authors contributed to the conceptualization and design, provided the analysis, and contributed to the manuscript prep-aration. Crane completed the data collection. Crane can be reached at cranes@iupui.edu, with copy to ONFEditor@ons.org. (Submitted October 2017. Accepted March 26, 2018.)
References
Agrawal, M., & Danis, M. (2002). End-of-life care for terminally ill participants in clinical research. Journal of Palliative Medicine, 5, 729–737. https://doi.org/10.1089/109662102320880552
Agrawal, M., & Emanuel, E.J. (2003). Ethics of phase 1 oncology studies: Reexamining the arguments and data. JAMA, 290, 1075–1082. https://doi.org/10.1001/jama.290.8.1075
Baker, J.N., Leek, A.C., Salas, H.S., Drotar, D., Noll, R., Rheingold, S.R., & Kodish, E.D. (2013). Suggestions from adolescents, young adults, and parents for improving informed consent in phase 1 pediatric oncology trials. Cancer, 119, 4154–4161. https://doi.org/10.1002/cncr.28335
Barnes, M.J., Pressey, J., Adams, J., Hensler, M.A., & Madan-Swain, A. (2014). Physician and nurse beliefs of phase 1 trials in pediatric oncology. Cancer Nursing, 37, E48–E52. https://doi.org/10.1097/ncc.0000000000000099
Barrera, M., D’Agostino, N., Gammon, J., Spencer, L., & Baruchel, S. (2005). Health-related quality of life and enrollment in phase 1 trials in children with incurable cancer. Palliative and Supportive Care, 3, 191–196.
Bautista, F., Di Giannatale, A., Dias-Gastellier, N., Fahd, M., Valteau-Couanet, D., Couanet, D., . . . Geoerger, B. (2015). Patients in pediatric phase I and early phase II clinical oncology trials at Gustave Roussy: A 13-year center experience. Journal of Pediatric Hematology/Oncology, 37, e102–e110. https://doi.org/10.1097/mph.0000000000000237
Beardsmore, S., & Fitzmaurice, N. (2002). Palliative care in paediatric oncology. European Journal of Cancer, 38, 1900–1910.
Berdel, W.E., Knopf, H., Fromm, M., Schick, H.D., Busch, R., Fink, U., . . . Rastetter, J. (1988). Influence of phase I early clinical trials on the quality of life of cancer patients. A pilot study. Anticancer Research, 8, 313–321.
Berg, S.L. (2007). Ethical challenges in cancer research in children. Oncologist, 12, 1336–1343. https://doi.org/10.1634/theoncologist.12-11-1336
Bluebond-Langner, M., Belasco, J.B., Goldman, A., & Belasco, C. (2007). Understanding parents’ approaches to care and treatment of children with cancer when standard therapy has failed. Journal of Clinical Oncology, 25, 2414–2419. https://doi.org/10.1200/JCO.2006.08.7759
Brock, K.E., Steineck, A., & Twist, C.J. (2016). Trends in end-of-life care in pediatric hematology, oncology, and stem cell transplant patients. Pediatric Blood and Cancer, 63, 516–522. https://doi.org/10.1002/pbc.25822
Broome, M.E., & Richards, D.J. (2003). The influence of relationships on children’s and adolescents’ participation in research. Nursing Research, 52, 191–197.
Campbell, S., & Whyte, F. (1999). The quality of life of cancer patients participating in phase I clinical trials using SEIQoL-DW. Journal of Advanced Nursing, 30, 335–343. https://doi.org/10.1046/j.1365-2648.1999.01079.x
Carlson, C., Reilly, M., & Hitchens, A. (2005). An innovative approach to the care of patients on phase I and phase II clinical trials: The role of the experimental therapeutics nurse. Journal of Pediatric Oncology Nursing, 22, 353–364. https://doi.org/10.1177/1043454205281763
Carlson, L., Ho, P., Smith, M., Reisch, J., & Weitman, S. (1996). Pediatric phase I drug tolerance: A review and comparison of recent adult and pediatric phase I trials. Journal of Pediatric Hematology/Oncology, 18, 250–256.
Carlson, L.E., Bultz, B.D., & Morris, D.G. (2005). Individualized quality of life, standardized quality of life, and distress in patients undergoing a phase I trial of the novel therapeutic Reolysin (reovirus). Health and Quality of Life Outcomes, 3, 7. https://doi.org/10.1186/1477-7525-3-7
Casarett, D.J., Karlawish, J.H., Henry, M.I., & Hirschman, K.B. (2002). Must patients with advanced cancer choose between a phase I trial and hospice? Cancer, 95, 1601–1604. https://doi.org/10.1002/cncr.10820
Cassel, J.B., Del Fabbro, E., Arkenau, T., Higginson, I.J., Hurst, S., Jansen, L.A., . . . Miller, F.G. (2016). Phase I cancer trials and palliative care: Antagonism, irrelevance, or synergy? Journal of Pain and Symptom Management, 52, 437–445. https://doi.org/10.1016/j.jpainsymman.2016.02.014
Chang, A. (2008). An exploratory survey of nurses’ perceptions of phase I clinical trials in pediatric oncology. Journal of Pediatric Oncology Nursing, 25, 14–23. https://doi.org/10.1177/1043454207311742
Cohen, M.Z., Slomka, J., Pentz, R.D., Flamm, A.L., Gold, D., Herbst, R.S., & Abbruzzese, J.L. (2007). Phase I participants’ views of quality of life and trial participation burdens. Supportive Care in Cancer, 15, 885–890. https://doi.org/10.1007/s00520-007-0216-0
Cousino, M.K., Zyzanski, S.J., Yamokoski, A.D., Hazen, R.A., Baker, J.N., Noll, R.B., . . . Kodish, E.D. (2012). Communicating and understanding the purpose of pediatric phase I cancer trials. Journal of Clinical Oncology, 30, 4367–4372. https://doi.org/10.1200/JCO.2012.42.3004
Cox, K. (1998). Investigating psychosocial aspects of participation in early anti-cancer drug trials: Towards a choice of methodology. Journal of Advanced Nursing, 27, 488–496.
Cox, K. (1999). Researching research: Patients’ experiences of participation in phase I and II anti-cancer drug trials. European Journal of Oncology Nursing, 3, 143–152. https://doi.org/10.1016/S1462-3889(99)80705-4
Cox, K. (2000). Enhancing cancer clinical trial management: Recommendations from a qualitative study of trial participants’ experiences. Psycho-Oncology, 9, 314–322.
Cox, K. (2002). The hopes of the dying: Examining patients’ experience of participation in early phase cancer clinical trials. Journal of Research in Nursing, 7, 60–73.
Cox, K. (2003). Assessing the quality of life of patients in phase I and II anti-cancer drug trials: Interviews versus questionnaires. Social Science and Medicine, 56, 921–934.
Cox, K., & Avis, M. (1996). Psychosocial aspects of participation in early anticancer drug trials. Report of a pilot study. Cancer Nursing, 19, 177–186.
Cox, K., Wilson, E., Arthur, A., Elkan, R., & Armstrong, S. (2005). A randomised controlled trial of nurse-managed trial conclusion following early phase cancer trial participation. British Journal of Cancer, 93, 41–45. https://doi.org/10.1038/sj.bjc.6602675
Crites, J., & Kodish, E. (2013). Unrealistic optimism and the ethics of phase I cancer research. Journal of Medical Ethics, 39, 403–406. https://doi.org/10.1136/medethics-2012-100752
Daugherty, C.K., Fitchett, G., Murphy, P.E., Peterman, A.H., Banik, D.M., Hlubocky, F., & Tartaro, J. (2005). Trusting God and medicine: Spirituality in advanced cancer patients volunteering for clinical trials of experimental agents. Psycho-Oncology, 14, 135–146. https://doi.org/10.1002/pon.829
de Vries, M.C., Houtlosser, M., Wit, J.M., Engberts, D.P., Bresters, D., Kaspers, G.J., & van Leeuwen, E. (2011). Ethical issues at the interface of clinical care and research practice in pediatric oncology: A narrative review of parents’ and physicians’ experiences. BMC Medical Ethics, 12, 18. https://doi.org/10.1186/1472-6939-12-18
Deatrick, J.A., Angst, D.B., & Moore, C. (2002). Parents’ views of their children’s participation in phase I oncology clinical trials. Journal of Pediatric Oncology Nursing, 19(4), 114–121. https://doi.org/10.1177/104345420201900402
Ekert, H. (2013). Can phase I cancer research studies in children be justified on ethical grounds? Journal of Medical Ethics, 39, 407. https://doi.org/10.1136/medethics-2012-101125
Estlin, E.J., Cotterill, S., Pratt, C.B., Pearson, A.D., & Bernstein, M. (2000). Phase I trials in pediatric oncology: Perceptions of pediatricians from the United Kingdom Children’s Cancer Study Group and the Pediatric Oncology Group. Journal of Clinical Oncology, 18, 1900–1905. https://doi.org/10.1200/JCO.2000.18.9.1900
Eton, D.T., Ramalho de Oliveira, D., Egginton, J.S., Ridgeway, J.L., Odell, L., May, C.R., & Montori, V.M. (2012). Building a measurement framework of burden of treatment in complex patients with chronic conditions: A qualitative study. Patient Related Outcome Measures, 3, 39–49. https://doi.org/10.2147/PROM.S34681
Finlay, E., Lu, H.L., Henderson, H.R., O’Dwyer, P.J., & Casarett, D.J. (2009). Do phase 1 patients have greater needs for palliative care compared with other cancer patients? Cancer, 115, 446–453. https://doi.org/10.1002/cncr.24025
Furman, W.L., Pratt, C.B., Rivera, G.K., Krischer, J.P., Kamen, B.A., & Vietti, T.J. (1989). Mortality in pediatric phase I clinical trials. Journal of the National Cancer Institute, 81, 1193–1194.
George, G.C., Iwuanyanwu, E.C., Anderson, K.O., Yusuf, A., Zinner, R.G., Piha-Paul, S.A., . . . Hong, D.S. (2016). Sleep quality and its association with fatigue, symptom burden, and mood in patients with advanced cancer in a clinic for early-phase oncology clinical trials. Cancer, 122, 3401–3409. https://doi.org/10.1002/cncr.30182
Gilliam, M.B., Madan-Swain, A., Adams, J.M., & Pressey, J.G. (2013). Physician perceptions and beliefs of phase I trials in pediatric oncology. Pediatric Blood and Cancer, 60, E67–E69. https://doi.org/10.1002/pbc.24522
Glaser, B.G., & Strauss, A.L. (1965). Awareness of dying: A study of social interaction. Chicago, IL: Aldine Publishing Company.
Glaser, B.G., & Strauss, A.L. (1968). Time for dying. Chicago, IL: Aldine Publishing Company.
Godskesen, T., Nygren, P., Nordin, K., Hansson, M., & Kihlbom, U. (2013). Phase 1 clinical trials in end-stage cancer: Patient understanding of trial premises and motives for participation. Supportive Care in Cancer, 21, 3137–3142. https://doi.org/10.1007/s00520-013-1891-7
Haase, J.E., Heiney, S.P., Ruccione, K.S., & Stutzer, C. (1999). Research triangulation to derive meaning-based quality-of-life theory: Adolescent resilience model and instrument development. International Journal of Cancer, 12(Suppl.), 125–131.
Haase, J.E., Kintner, E.K., Monahan, P.O., & Robb, S.L. (2014). The resilience in illness model, part 1: Exploratory evaluation in adolescents and young adults with cancer. Cancer Nursing, 37, E1–E12. https://doi.org/10.1097/NCC.0b013e31828941bb
Haase, J.E., Kintner, E.K., Robb, S.L., Stump, T.E., Monahan, P.O., Phillips, C., . . . Burns, D.S. (2017). The resilience in illness model part 2: Confirmatory evaluation in adolescents and young adults with cancer. Cancer Nursing, 40, 454–463. https://doi.org/10.1097/NCC.0000000000000450
Hazen, R.A., Zyzanski, S., Baker, J.N., Drotar, D., & Kodish, E. (2015). Communication about the risks and benefits of phase I pediatric oncology trials. Contemporary Clinical Trials, 41, 139–145. https://doi.org/10.1016/j.cct.2015.01.015
Helft, P.R., Hlubocky, F., Wen, M., & Daugherty, C.K. (2003). Associations among awareness of prognosis, hopefulness, and coping in patients with advanced cancer participating in phase I clinical trials. Supportive Care in Cancer, 11, 644–651. https://doi.org/10.1007/s00520-003-0496-y
Hinds, P.S., Drew, D., Oakes, L.L., Fouladi, M., Spunt, S.L., Church, C., & Furman, W.L. (2005). End-of-life care preferences of pediatric patients with cancer. Journal of Clinical Oncology, 23, 9146–9154. https://doi.org/10.1200/JCO.2005.10.538
Hinds, P.S., Oakes, L., Furman, W., Foppiano, P., Olson, M.S., Quargnenti, A., . . . Strong, C. (1997). Decision making by parents and healthcare professionals when considering continued care for pediatric patients with cancer. Oncology Nursing Forum, 24, 1523–1528.
Hinds, P.S., Oakes, L.L., Hicks, J., Powell, B., Srivastava, D.K., Spunt, S.L., . . . Furman, W.L. (2009). “Trying to be a good parent” as defined by interviews with parents who made phase I, terminal care, and resuscitation decisions for their children. Journal of Clinical Oncology, 27, 5979–5985. https://doi.org/10.1200/jco.2008.20.0204
Hui, D., Parsons, H., Nguyen, L., Palla, S.L., Yennurajalingam, S., Kurzrock, R., & Bruera, E. (2010). Timing of palliative care referral and symptom burden in phase 1 cancer patients: A retrospective cohort study. Cancer, 116, 4402–4409. https://doi.org/10.1002/cncr.25389
Hutchison, C. (1998). Phase I trials in cancer patients: Participants’ perceptions. European Journal of Cancer Care, 7, 15–22.
Jansen, L.A., Mahadevan, D., Appelbaum, P.S., Klein, W.M., Weinstein, N.D., Mori, M., . . . Sulmasy, D.P. (2016). Dispositional optimism and therapeutic expectations in early-phase oncology trials. Cancer, 122, 1238–1246.
Kapo, J., & Casarett, D. (2002). Palliative care in phase 1 trials: An ethical obligation or undue inducement? Journal of Palliative Medicine, 5, 661–665. https://doi.org/10.1089/109662102320880462
Kearns, P., & Morland, B. (2014). New drug development in childhood cancer. Current Opinion in Pediatrics, 26, 37–42. https://doi.org/10.1097/mop.0000000000000054
Kelly, K.P., Hooke, M.C., Ruccione, K., Lanier, W., & Haase, J. (2014). Children’s Oncology Group nursing research framework. Seminars in Oncology Nursing, 30, 17–25. https://doi.org/10.1016/j.soncn.2013.12.004
Kessler, E.R., Moss, A., Eckhardt, S.G., Laudenslager, M.L., Kilbourn, K., Mauss, I.B., . . . Kutner, J.S. (2014). Distress among caregivers of phase I trial participants: A cross-sectional study. Supportive Care in Cancer, 22, 3331–3340. https://doi.org/10.1007/s00520-014-2380-3
Kim, A., Fox, E., Warren, K., Blaney, S.M., Berg, S.L., Adamson, P.C., . . . Widemann, B.C. (2008). Characteristics and outcome of pediatric patients enrolled in phase I oncology trials. Oncologist, 13, 679–689. https://doi.org/10.1634/theoncologist.2008-0046
Kvale, E.A., Woodby, L., & Williams, B.R. (2010). The experience of older patients with cancer in phase 1 clinical trials: A qualitative case series. American Journal of Hospice and Palliative Care, 27, 474–481. https://doi.org/10.1177/1049909110365072
Landier, W., Leonard, M., & Ruccione, K.S. (2013). Children’s Oncology Group’s 2013 blueprint for research: Nursing discipline. Pediatric Blood and Cancer, 60, 1031–1036. https://doi.org/10.1002/pbc.24415
Langenberg, S.M., Peters, M.E., van der Graaf, W.T., Wymenga, A.N., Prins, J.B., & van Herpen, C.M. (2016). How did partners experience cancer patients’ participation in a phase I study? An observational study after a patient’s death. Palliative and Supportive Care, 14, 241–249. https://doi.org/10.1017/S1478951515000887
Lansky, S.B., List, M.A., Lansky, L.L., Ritter-Sterr, C., & Miller, D.R. (1987). The measurement of performance in childhood cancer patients. Cancer, 60, 1651–1656.
Last, B.F. (1992). The phenomenon of double protection. In B.F. Last, & A.M. van Veldhuizen (Eds.), Developments in pediatric psychosocial oncology (pp. 39–51). Lisse, Amsterdam: Swets & Zeitlinger.
Lee, D.P., Skolnik, J.M., & Adamson, P.C. (2005). Pediatric phase I trials in oncology: An analysis of study conduct efficiency. Journal of Clinical Oncology, 23, 8431–8441. https://doi.org/10.1200/jco.2005.02.1568
Levine, D.R., Johnson, L.M., Mandrell, B.N., Yang, J., West, N.K., Hinds, P.S., & Baker, J.N. (2015). Does phase 1 trial enrollment preclude quality end-of-life care? Phase 1 trial enrollment and end-of-life care characteristics in children with cancer. Cancer, 121, 1508–1512. https://doi.org/10.1002/cncr.29230
Mack, C.H. (1998). The quest for treatment: Cancer patients’ experience of Phase I clinical trials. Los Angeles, CA: The University of California.
Mack, J.W., Joffe, S., Hilden, J.M., Watterson, J., Moore, C., Weeks, J.C., & Wolfe, J. (2008). Parents’ views of cancer-directed therapy for children with no realistic chance for cure. Journal of Clinical Oncology, 26, 4759–4764. https://doi.org/10.1200/JCO.2007.15.6059
Marshall, P.A., Magtanong, R.V., Leek, A.C., Hizlan, S., Yamokoski, A.D., & Kodish, E.D. (2012). Negotiating decisions during informed consent for pediatric phase I oncology trials. Journal of Empirical Research on Human Research Ethics, 7, 51–59. https://doi.org/10.1525/jer.2012.7.2.51
Maurer, S.H., Hinds, P.S., Spunt, S.L., Furman, W.L., Kane, J.R., & Baker, J.N. (2010). Decision making by parents of children with incurable cancer who opt for enrollment on a phase I trial compared with choosing a do not resuscitate/terminal care option. Journal of Clinical Oncology, 28, 3292–3298. https://doi.org/10.1200/jco.2009.26.6502
Melink, T.J., Clark, G.M., & Von Hoff, D.D. (1992). The impact of phase I clinical trials on the quality of life of patients with cancer. Anti-Cancer Drugs, 3, 571–576.
Meyers, F.J., Linder, J., Beckett, L., Christensen, S., Blais, J., & Gandara, D.R. (2004). Simultaneous care: A model approach to the perceived conflict between investigational therapy and palliative care. Journal of Pain and Symptom Management, 28, 548–556. https://doi.org/10.1016/j.jpainsymman.2004.03.002
Miller, F.G., & Joffe, S. (2008). Benefit in phase 1 oncology trials: Therapeutic misconception or reasonable treatment option? Clinical Trials, 5, 617–623. https://doi.org/10.1177/1740774508097576
Miller, V.A., Baker, J.N., Leek, A.C., Drotar, D., & Kodish, E. (2014). Patient involvement in informed consent for pediatric phase I cancer research. Journal of Pediatric Hematology/Oncology, 36, 635–640. https://doi.org/10.1097/mph.0000000000000112
Miller, V.A., Baker, J.N., Leek, A.C., Hizlan, S., Rheingold, S.R., Yamokoski, A.D., . . . Kodish, E. (2013). Adolescent perspectives on phase I cancer research. Pediatric Blood and Cancer, 60, 873–878. https://doi.org/10.1002/pbc.24326
Miller, V.A., Cousino, M., Leek, A.C., & Kodish, E.D. (2014). Hope and persuasion by physicians during informed consent for phase I trials. Journal of Clinical Oncology, 32, 3229–3235. https://doi.org/10.1200/JCO.2014.55.2588
Moore, S. (2001). A need to try everything: Patient participation in phase I trials. Journal of Advanced Nursing, 33, 738–747. https://doi.org/10.1046/j.1365-2648.2001.01715.x
Mor, V., Laliberte, L., Morris, J.N., & Wiemann, M. (1984). The Karnofsky Performance Status Scale: An examination of its reliability and validity in a research setting. Cancer, 53, 2002–2007.
Morgenstern, D.A., Hargrave, D., Marshall, L.V., Gatz, S.A., Barone, G., Crowe, T., . . . Moreno, L. (2014). Toxicity and outcome of children and adolescents participating in phase I/II trials of novel anticancer drugs: The Royal Marsden experience. Journal of Pediatric Hematology/Oncology, 36, 218–223. https://doi.org/10.1097/mph.0000000000000003
Oberman, M., & Frader, J. (2003). Dying children and medical research: Access to clinical trials as benefit and burden. American Journal of Law and Medicine, 29, 301–317.
Oppenheim, D., Geoerger, B., & Hartmann, O. (2005). Ethical issues in pediatric oncology: Phase I-II trials based on a mother’s point of view. Bulletin du Cancer, 92, E57–E60.
Paoletti, X., Geoerger, B., Doz, F., Baruchel, A., Lokiec, F., & Le Tourneau, C. (2013). A comparative analysis of paediatric dose-finding trials of molecularly targeted agent with adults’ trials. European Journal of Cancer, 49, 2392–2402. https://doi.org/10.1016/j.ejca.2013.02.028
Rouanne, M., Massard, C., Hollebecque, A., Rousseau, V., Varga, A., Gazzah, A., . . . Soria, J.C. (2013). Evaluation of sexuality, health-related quality-of-life and depression in advanced cancer patients: A prospective study in a phase I clinical trial unit of predominantly targeted anticancer drugs. European Journal of Cancer, 49, 431–438. https://doi.org/10.1016/j.ejca.2012.08.008
Sav, A., Kendall, E., McMillan, S.S., Kelly, F., Whitty, J.A., King, M.A., & Wheeler, A.J. (2013). ‘You say treatment, I say hard work’: Treatment burden among people with chronic illness and their carers in Australia. Health and Social Care in the Community, 21, 665–674. https://doi.org/10.1111/hsc.12052
Sav, A., King, M.A., Whitty, J.A., Kendall, E., McMillan, S.S., Kelly, F., . . . Wheeler, A.J. (2013). Burden of treatment for chronic illness: A concept analysis and review of the literature. Health Expectations, 18, 312–324. https://doi.org/10.1111/hex.12046
Schou, K.C. (1992). Awareness contexts and the construction of dying in the cancer treatment setting: ‘Micro’ and ‘macro’ levels in narrative analysis. Sociological Review, 40(S1), 238–263. https://doi.org/10.1111/j.1467-954X.1992.tb03395.x
Shah, S., Weitman, S., Langevin, A.M., Bernstein, M., Furman, W., & Pratt, C. (1998). Phase I therapy trials in children with cancer. Journal of Pediatric Hematology/Oncology, 20, 431–438.
Siegel, R.L., Miller, K.D., & Jemal, A. (2016). Cancer statistics, 2016. CA: A Cancer Journal for Clinicians, 66, 7–30.
Stetz, K.M. (1993). Survival work: The experience of the patient and the spouse involved in experimental treatment for cancer. Seminars in Oncology Nursing, 9, 121–126.
Sun, V., Cooke, L., Chung, V., Uman, G., Smith, T.J., & Ferrell, B. (2014). Feasibility of a palliative care intervention for cancer patients in phase I clinical trials. Journal of Palliative Medicine, 17, 1365–1368. https://doi.org/10.1089/jpm.2014.0108
Tomlinson, D., Bartels, U., Gammon, J., Hinds, P.S., Volpe, J., Bouffet, E., . . . Sung, L. (2011). Chemotherapy versus supportive care alone in pediatric palliative care for cancer: Comparing the preferences of parents and health care professionals. Canadian Medical Association Journal, 183, E1252–E1258. https://doi.org/10.1503/cmaj.110392
Ulrich, C.M., Grady, C., & Wendler, D. (2004). Palliative care: A supportive adjunct to pediatric phase I clinical trials for anticancer agents? Pediatrics, 114, 852–855.
Ulrich, C.M., Knafl, K.A., Ratcliffe, S.J., Richmond, T.S., Grady, C., Miller-Davis, C., & Wallen, G.R. (2012). Developing a model of the benefits and burdens of research participation in cancer clinical trials. AJOB Primary Research, 3(2), 10–23. https://doi.org/10.1080/21507716.2011.653472
Weber, J.S., Levit, L.A., Adamson, P.C., Bruinooge, S., Burris, H.A., IV, Carducci, M.A., . . . Schuchter, L.M. (2015). American Society of Clinical Oncology policy statement update: The critical role of phase I trials in cancer research and treatment. Journal of Clinical Oncology, 33, 278–284. https://doi.org/10.1200/JCO.2014.58.2635
Weinfurt, K.P., Seils, D.M., Lin, L., Sulmasy, D.P., Astrow, A.B., Hurwitz, H.I., . . . Meropol, N.J. (2012). Research participants’ high expectations of benefit in early-phase oncology trials: Are we asking the right question? Journal of Clinical Oncology, 30, 4396–4400. https://doi.org/10.1200/jco.2011.40.6587
Weisman, A.D. (1972). On dying and denying: A psychiatric study of terminality. Pasadena, CA: Behavioral Publications.
Whittemore, R., & Knafl, K. (2005). The integrative review: Updated methodology. Journal of Advanced Nursing, 52, 546–553. https://doi.org/10.1111/j.1365-2648.2005.03621.x
Wilson, E., Cox, K., & Elkan, R. (2005). Enhancing cancer trial management: an intervention study of the impact of providing information, trial results and support to patients in phase I and II anti-cancer drug trials at trial conclusion. Clinical Effectiveness in Nursing, 9, 119–132. https://doi.org/10.1016/j.cein.2006.06.003
Wootten, A.C., Abbott, J.M., Siddons, H.M., Rosenthal, M.A., & Costello, A.J. (2011). A qualitative assessment of the experience of participating in a cancer-related clinical trial. Supportive Care in Cancer, 19, 49–55. https://doi.org/10.1007/s00520-009-0787-z
World Medical Association. (2013). World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA, 310, 2191–2194. https://doi.org/10.1001/jama.2013.281053
Yoder, L.H., O’Rourke, T.J., Etnyre, A., Spears, D.T., & Brown, T.D. (1997). Expectations and experiences of patients with cancer participating in phase I clinical trials. Oncology Nursing Forum, 24, 891–896.
Ziaian, T., Sawyer, M.G., Reynolds, K.E., Carbone, J.A., Clark, J.J., Baghurst, P.A., . . . French, D.J. (2006). Treatment burden and health-related quality of life of children with diabetes, cystic fibrosis and asthma. Journal of Paediatrics and Child Health, 42, 596–600. https://doi.org/10.1111/j.1440-1754.2006.00943.x




