Examining Adherence With Recommendations for Follow-Up in the Prevention Among Colorectal Cancer Survivors Study
Purpose/Objectives: To explore the impact of health professionals’ recommendations for medical follow-up among colorectal cancer (CRC) survivors.
Design: Cross-sectional survey.
Setting: Mailed surveys and telephone interviews with CRC survivors in California.
Sample: 593 adults diagnosed with a primary CRC six to seven years before the time of the study.
Methods: Participants were identified through California Cancer Registry records and invited to take part in a survey delivered via mail or through telephone interview.
Main Research Variables: The survey assessed cancer history, current preventive health practices, health status, demographics, and other cancer-related experiences.
Findings: More than 70% of CRC survivors received recommendations for routine checkups, surveillance colonoscopy, or other cancer screenings after completing CRC treatment, and 18%–22% received no such recommendations. Recommendations were sometimes given in writing. Receiving a recommendation for a specific type of follow-up was associated with greater adherence to corresponding guidelines for routine checkups, colonoscopy, mammography, and Papanicolaou testing. Receiving written (versus unwritten) recommendations led to greater adherence only for colonoscopy.
Conclusions: Most CRC survivors reported receiving recommendations for long-term medical follow-up and largely adhered to guidelines for follow-up. Receiving a health professional’s recommendation for follow-up was consistently associated with patient adherence, and limited evidence showed that recommendations in written form led to greater adherence than unwritten recommendations.
Implications for Nursing: Given the increasingly important role of the oncology nurse in survivorship care, nurses can be instrumental in ensuring appropriate surveillance and follow-up care among CRC survivors. Conveying recommendations in written form, as is done in survivorship care plans, may be particularly effective.
Jump to a section
People diagnosed with colorectal cancer (CRC) are now living longer after diagnosis than they did 30 years ago (Howlader et al., 2013), but heightened risks for cancer recurrence, second primary cancers, and other health problems are a concern during this lengthened phase of survivorship. To manage these risks and facilitate early detection of subsequent disease, cancer survivors are advised to follow a schedule of routine medical checkups, screenings, and surveillance (Desch et al., 2005; National Comprehensive Cancer Network [NCCN], 2012).
Compared with non-cancer controls, cancer survivors generally report higher rates of recommended cancer screenings (Bellizzi, Rowland, Jeffery, & McNeel, 2005; Fairley, Hawk, & Pierre, 2006; Hudson et al., 2009; Trask et al., 2005). However, a systematic review on post-treatment surveillance of CRC survivors concluded that 20%–49% of survivors are nonadherent with recommendations for surveillance colonoscopy at five years postdiagnosis, and as many as 23% of survivors attend fewer than the number of recommended office visits (Carpentier, Vernon, Bartholomew, Murphy, & Bluethmann, 2013). Given the potential health benefits (e.g., increased survival) associated with undergoing recommended surveillance after CRC treatments (Figueredo et al., 2003; Jeffery, Hickey, & Hider, 2007; Renehan, Egger, Saunders, & O’Dwyer, 2002; Tjandra & Chan, 2007), underuse of appropriate follow-up is an issue of growing public health concern.
Research has revealed that CRC survivors who are African American, under- or uninsured, and who have more comorbidities are among the least likely to undergo post-treatment surveillance (Carpentier et al., 2013; Hudson et al., 2009; Rolnick et al., 2005; Rulyak, Mandelson, Brentnall, Rutter, & Wagner, 2004). Other characteristics have been inconsistently reported across studies, and a need remains to identify modifiable factors associated with follow-up care for CRC survivors.
A potential driver of appropriate follow-up care after cancer involves recommendations for follow-up in the form of survivorship care plans (SCPs). In From Cancer Patient to Cancer Survivor: Lost in Transition, the Institute of Medicine (Hewitt, Greenfield, & Stovall, 2006) recommended that every patient with cancer who is completing primary treatment be provided with a comprehensive treatment summary and a written follow-up plan, referred to as an SCP, that details evidence-based standards of care for protecting health after cancer. According to the IOM, SCPs should include written recommendations for medical follow-up and surveillance to be performed routinely after treatment ceases, detailing how often and where survivors should be seen for their visits and screening tests. Although the IOM report received widespread acclaim and launched a nationwide call for SCPs, progress has been slow, and many cancer survivors continue to report never receiving written documents or materials resembling SCPs (Jabson & Bowen, 2013; Sabatino et al., 2013). The slow rate of SCP uptake has driven investigations on the barriers to developing and delivering SCPs, which have uncovered a number of logistical challenges in implementing the IOM recommendations (Dulko et al., 2013). There is a lack of evidence demonstrating the efficacy of SCPs to affect care received or survivor outcomes post-treatment. Only a few studies have directly examined this issue, one of which used National Health Interview Survey (NHIS) data and found that receiving written instructions for follow-up was associated with receiving provider recommendations for surveillance among breast and cervical cancer survivors, but not with actual follow-up completion (Sabatino et al., 2013). However, Commission on Cancer ([COC], 2012) standards required that all COC-accredited cancer programs began delivering SCPs to increasing proportions of eligible patients beginning in 2015.
While research among general, non-cancer populations has demonstrated that a health professional’s recommendation for screening is a powerful influence on actual screening behavior (Brawarsky, Brooks, Mucci, & Wood, 2004; Guessous et al., 2010; Subramanian, Klosterman, Amonkar, & Hunt, 2004), scant evidence exists of this phenomenon among cancer survivors. An assumption underlying the push for SCP use is that recommendations for medical follow-up in the form of SCPs will lead to greater adherence with guidelines for follow-up; however, little evidence has been reported to support this presumption. Therefore, the goal of the current study was to examine the association between health professionals’ recommendations for follow-up and actual follow-up received by CRC survivors in a population-based sample.
The Prevention Among Colorectal Cancer Survivors (PACCS) study by the Centers for Disease Control and Prevention (CDC) was initiated to assess the health status, health behaviors, and medical follow-up of CRC survivors more than five years after diagnosis and to identify barriers to practicing healthy behaviors and receiving recommended follow-up after treatment for CRC. This population-based sample was used in the current study to examine survivors’ self-reported follow-up care as a function of recommendations made to them by health professionals. The authors also examined the method in which recommendations were given, whether they were in written form or not. The authors expected that any recommendations received by survivors would improve adherence to follow-up care and that this relationship would be stronger if recommendations were given in writing.
Methods
Participants for the PACCS study were recruited through the California Cancer Registry (CCR), operated in early 2010 by the Public Health Institute (PHI). ICF International, a company that provides research and evaluation services for government and businesses, served as CDC’s contractor for the study. All recruitment and study methods were approved by the institutional review boards of four organizations (CDC, ICF International, PHI, and CCR). CCR ascertained from their records a sampling frame of 11,168 cases of CRC diagnosed in California from 2003–2004. These sampling years were chosen to identify cancer survivors diagnosed more than five years prior to the time of planned data collection. Within this sampling frame, they applied the initial study eligibility criteria, which included (a) having a primary cancer in a localized or regional stage, (b) having no previous cancer diagnosis, (c) being a resident of California at the time of diagnosis, (d) being aged 18 years or older at the time of diagnosis, (e) having a current vital status, (f) not having been contacted in the past 12 months to participate in any other studies using the CCR database, and (g) having no “do not contact” flag on record. Of the 11,168 cases, 10,315 met the initial eligibility criteria and were used as the sampling pool. The authors stratified eligible cases by race and ethnicity groups and oversampled minority racial and ethnic groups to obtain more accurate population estimates. A total of 1,920 cases were then randomly selected for recruitment, and additional eligibility criteria were applied. Survivors were ineligible if they were unable to complete the survey in English or because of mental or extreme physical incompetence (see Figure 1). PHI sent 1,781 advance letters to potentially eligible survivors who had not been excluded before or during the process of physician notification.
Recruitment efforts followed the Tailored Design Method (Dillman, 2007), which consisted of mailing, sequentially, an initial survey packet, a reminder postcard at two weeks, and a follow-up survey packet at four weeks. Nonresponders were contacted by telephone and invited to complete the survey via telephone interview. The survey packet included a cover letter, informed consent document, study questionnaire, $10 incentive, pen, and a pre-addressed, postage-paid envelope for returning the questionnaire. All recruitment and data collection took place in early 2010.
Questionnaire Content
The PACCS survey contained previously validated measures and scales, as well as novel questions developed for the study. The present analysis used the following variables: past and current health, medical follow-up, recommendations, and demographics.
Questions were asked to determine the method of diagnosis and any current cancer treatments. Survivors undergoing active treatment for any cancer were excluded from the analysis. General health status was measured using a five-point Likert-type scale.
Survivors were also asked how long it had been since their last colonoscopy, routine checkup, mammogram (females only), and cervical screening by Papanicolaou (Pap) test (females only). Questions and closed-ended responses were modified from the cancer control module of the NHIS.
Participants were asked if, since completing treatment, they had received guidance from a doctor, nurse, or other health professional about getting medical follow-up, including routine checkups, colonoscopy, mammogram, and Pap testing. Participants who answered “yes” were asked whether the guidance had been written or printed for them. The authors categorized follow-up instructions as written, unwritten, and none. Similar questions were included on the cancer control module of the NHIS and have been categorized similarly (Sabatino et al., 2013). Standard questions were asked about age, gender, race, ethnicity, marital status, educational attainment, employment status, and health insurance.
Defining Adherence With Recommendations
Literature reviews were conducted in 2008 and during data collection in 2010 to determine current recommendations for surveillance colonoscopy, routine checkups, and cancer screening by mammography and Pap testing. Recommendations specific to CRC survivors were available for colonoscopy and routine checkups, and recommendations for cervical and breast cancer screening were adopted from those developed for the general public. Adherence with follow-up for CRC survivors five to seven years postdiagnosis was based on guidelines from the NCCN and the American Cancer Society (2005) (Rex et al., 2006), the American Society of Clinical Oncology (Desch et al., 2005), the American Gastroenterological Association (Winawer et al., 2003), and the U.S. Preventive Services Task Force (2013). Survivors were considered adherent with surveillance colonoscopy if they had undergone colonoscopy within the past three years, with routine checkups if they had been seen within the past six months, with mammography (among women aged 40 years or older) if they had had one within the past two years, and with cervical screening (for women younger than age 65 years with no previous hysterectomy) if they had received a Pap test within the past three years.
Statistical Analysis
The authors calculated base weights for the race and ethnicity totals that were equal to the inverse selection probability. The final weights were then obtained by poststratification, which better reflected the racial and age distribution in the survivor population. Descriptive statistics were used to examine demographic characteristics, receipt of recommendations for checkups and screening, and adherence to recommendations. Associations were assessed between survivor characteristics and adherence to recommendations for each screening test and checkup with the Rao-Scott chi-square test (Rao & Scott, 1987). Differences between written and unwritten recommendations were examined with linear contrasts separately for checkups and each screening type. To improve the reliability of the estimates, the authors dichotomized the health status variable to excellent, very good, or good versus fair or poor and insurance status to none, public assistance only, Medicare, or Medicaid versus private or military.
Multivariable logistic regression models were used to calculate adjusted percentage estimates (predicted margins) (Graubard & Korn, 1999) and their differences and ratios, and to examine the association between receiving a recommendation and undergoing routine checkups, colonoscopy, and mammography after controlling for other survivor characteristics. Cervical screening estimates were not included in this analysis because of insufficient numbers. The survivor characteristics that the authors controlled included gender (when applicable), education, insurance status, marital status, and health status. Only variables significantly associated (p < 0.05) with their respective outcome were left in the model. For this analysis, the authors further dichotomized the following variables: education (less than some college versus some college or more) and marital status (married or living together versus divorced, widowed, separated, or never married).
To generalize the results to the population of CRC survivors within the state of California, data were analyzed with SAS® and SUDAAN, version 10, which computed weighted population estimates and 95% confidence intervals (CIs) and accounted for the complex survey design and nonresponse.
Results
Response rates were based on 1,781 cases contacted for the study via a mailed letter sent in advance of the survey packet. Following the advance letter, the sampling pool decreased by those who refused participation (n = 84), were deemed ineligible (n = 105), and were found to have invalid addresses with no additional contact information (n = 179). Survey packets were then mailed to 1,414 survivors, which yielded 593 completed surveys (582 returned by mail; 11 conducted via telephone interview). The unadjusted response rate (44%) was calculated as the number of completed surveys divided by the number of potentially eligible cases in the physician-consented sample, which included all refusals, incomplete responses, and nonresponders. Because this rate assumes every instance of nonresponse represents an eligible participant, the authors also calculated an adjusted response rate (46%), which estimates the same proportion of eligibility (85%) in nonresponse cases as in the cases with which contact was made. A cooperation rate of 64% was calculated as the proportion of eligible participants with whom contact was made who agreed to participate. One participant with missing race and ethnicity information was excluded from the analysis because weights could not be calculated.
Survivors were, on average, 6.2 years past their primary diagnosis of CRC (range = 5.2–7.2 years). Equal proportions of males and females participated, the mean age was 73.8 years, and 65% were Non-Hispanic Caucasian (see Table 1). More than 60% had some college education or had completed college. The vast majority were married or living with a partner and reported their health as good, very good, or excellent. About 60% were insured through Medicare, Medicaid, or public assistance only. 
A majority of participants (71%–76%) received written or unwritten recommendations for routine checkups, surveillance colonoscopy, and other cancer screenings; however, 18%–22% received no such guidance (see Figure 2). Although 44% and 58% received recommendations in writing for Pap testing and mammography, respectively, 28% and 13% received only unwritten recommendations for Pap testing and mammography, respectively. 
Adherence to guidelines for routine checkups was 90% (95% CI [87.5, 93]), and adherence with surveillance colonoscopy was 69% (95% CI [65, 73.7]). Among those eligible, 86% (95% CI [77.4, 94]) were adherent with recommendations for Pap testing, and 71% (95% CI [64.8, 77]) were adherent with recommendations for mammography.
Survivors with Medicare, Medicaid, public assistance only, or no insurance were less likely to be adherent to the schedule of recommended routine checkups (88%) than were those with private or military insurance (94%) (p = 0.046). A significant association was observed between general health and adherence to colonoscopy guidelines (p = 0.035), where survivors with fair or poor health had lower adherence with colonoscopy (59%) than those with good to excellent health (72%). Adherence to recommendations for mammography among survivors with poor health was lower (49%, 95% CI [35.4, 62.8]) than adherence among those with good to excellent health (78%, 95% CI [72, 84.7]) (see Table 2).
Adherence was associated with a health professional’s recommendation for each type of follow-up. For routine checkups and each of the screening tests, those not having received a recommendation were less likely to be up to date with them than those having received a written or unwritten recommendation (p < 0.05 for checkups, p < 0.001 for each screening test). However, receiving a written recommendation rather than an unwritten one was only significantly associated with higher adherence for colonoscopy (p < 0.05). Although adherence with cervical screening recommendations for eligible women younger than age 65 years increased from 48% to more than 96% given a recommendation for that test (p < 0.001), these results should be interpreted with caution because of the small number of women who did not receive recommendations.
Further analysis using multivariable models revealed that, after controlling for demographic variables, differences in the adjusted percentages of adherence varied by type of recommendation (any recommendation versus no recommendation) from 10% (p = 0.016) for checkups to 41% (p < 0.001) for colonoscopy and 59% (p < 0.001) for mammography. Associations between recommendations and adherence to these recommendations varied in a similar manner with decreasing prevalence ratios. Survivors with any recommendation were more likely to adhere to the recommendation than those with no recommendation for routine checkup (adjusted prevalence ratio [aPR] = 0.89, p = 0.004), colonoscopy (aPR = 0.48, p < 0.001), and mammography (aPR = 0.3, p < 0.001).
Discussion
Efforts to improve survivors’ long-term health have increasingly focused on the importance of medical follow-up and early detection of subsequent disease. The field of oncology nursing is undergoing similar shifts in focus, with expanded emphases on the stage of care beyond active treatment, including activities related to increasing adherence with recommended cancer prevention, detection, and surveillance. In this group of CRC survivors six to seven years after diagnosis, adherence with recommended routine checkups was high (90%), but adherence with surveillance colonoscopy was substantially lower (69%), which is consistent with a review by Carpentier et al. (2013) on the surveillance patterns of CRC survivors. However, the current study went a step further in analyzing these follow-up patterns in light of health professionals’ recommendations, an area in which oncology nurses can play a critical role.
By recruiting survivors through a cancer registry, the authors were able to present population-based estimates from a diverse group of CRC survivors. The findings of the current study go beyond previous research that has identified sociodemographic characteristics associated with CRC survivors’ medical follow-up (Carpentier et al., 2013; Rolnick et al., 2005; Rulyak et al., 2004) because the current study reveals a modifiable influence on follow-up care. The findings suggest that health professionals’ recommendations for medical follow-up are significantly related to actual follow-up behavior and, therefore, are of paramount importance to the early detection of recurrence, polyps, and precancerous lesions.
About one-fifth of survivors received no recommendations for medical follow-up after CRC treatment had ended, but the majority received recommendations, and more than half of those received them in written form. Whereas receiving an unwritten or written recommendation were both associated with greater adherence to guidelines for routine checkups, colonoscopy, mammography, and Pap testing, receiving a written recommendation rather than an unwritten one appeared to offer an additional boost to adherence with colonoscopy. Of the variables examined as possible covariates of follow-up, a health professional’s recommendation demonstrated the strongest association with adherence.
Examining factors associated with follow-up after CRC treatment is an important step in identifying ways to improve the long-term health of survivors. SCPs have been advocated as a means by which survivors can become informed of recommendations for long-term follow-up and are intended to serve the role of a direct and tangible recommendation from the healthcare team. Although the full impact of SCPs will not be known until many years after they become common practice, results from the current study indicate that recommendations included in SCPs are likely to increase medical follow-up and surveillance. Further research is needed to examine the impact of SCPs using prospective designs that compare different modes of delivery and evaluate the costs, barriers, and benefits of full-scale implementation. With the new COC standards, which require multifaceted change in survivorship care, comes a wealth of new research opportunities for evaluating the process and outcomes of these new components of care. Best practices will need to be tested and identified regarding the process of communicating information about follow-up to survivors and others closely involved; coordinating medical follow-up between survivors and healthcare professionals; and referring survivors to survivorship resources in the cancer center, community, and online (Grant, Economou, & Ferrell, 2010). With each change that is made to standard survivorship care, assessing the equity of support services to survivors from different backgrounds and the effectiveness of services offered will be important.
Limitations
Although a population-based sampling frame was used, the adjusted response rate was 46%, and the potential for response bias cannot be ruled out. However, the cooperation rate (i.e., proportion of those with whom the authors made contact who agreed to participate) was higher (64%). Although recruiting survivors from a cancer registry allowed for a diverse sample, the cross-sectional sampling and self-report methods prevented the verification of medical follow-up. Error was also possible in the degree to which participants recalled receiving recommendations in under- and overreporting. However, previous research that has compared CRC screening history via self-report versus medical record has found that self-report is largely reliable, particularly with regard to colonoscopy (Jones, Mongin, Lazovich, Church, & Yeazel, 2008; Khoja, McGregor, & Hilsden, 2007; Partin et al., 2008).
Implications for Nursing
In addition to traditional roles in coordinating and providing cancer care, oncology nurses can play an important role in facilitating the transition of patient with cancer to survivor and will likely have increasingly critical responsibilities related to communicating recommendations for follow-up care. The responsibilities for creating and delivering SCPs will, in many instances, reside with oncology nurses, nurse navigators, and other care providers meeting the COC requirements surrounding SCP delivery, further elevating the oncology nurse’s role in survivorship care. Future research is needed to examine the impact of activities and answer questions related to the nurse’s expanding role in developing and delivering SCPs.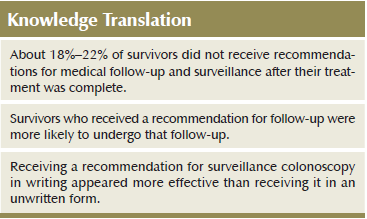
Conclusions
Six to seven years after a CRC diagnosis, the authors found that 18%–22% of survivors had not received any recommendations for follow-up care and surveillance. Those who did receive a recommendation for follow-up were more likely to undergo that follow-up, and receiving a recommendation for colonoscopy in writing appeared more influential than receiving it in an unwritten form. Successful communication regarding medical follow-up after treatment is essential to optimizing health after cancer, and the oncology nurse can play a pivotal role in the communication of this information. As the field of long-term survivorship care progresses, nurses must continue to monitor the health behaviors of survivors and look for opportunities to encourage appropriate follow-up and prevention practices.
References
Bellizzi, K.M., Rowland, J.H., Jeffery, D.D., & McNeel, T. (2005). Health behaviors of cancer survivors: Examining opportunities for cancer control intervention. Journal of Clinical Oncology, 23, 8884–8893. doi:10.1200/JCO.2005.02.2343
Brawarsky, P., Brooks, D.R., Mucci, L.A., & Wood, P.A. (2004). Effect of physician recommendation and patient adherence on rates of colorectal cancer testing. Cancer Detection and Prevention, 28, 260–268. doi:10.1016/j.cdp.2004.04.006
Carpentier, M.Y., Vernon, S.W., Bartholomew, L.K., Murphy, C.C., & Bluethmann, S.M. (2013). Receipt of recommended surveillance among colorectal cancer survivors: A systematic review. Journal of Cancer Survivorship, 7, 464–483. doi:10.1007/s11764-013-0290-x
Commission on Cancer. (2012). Cancer program standards 2012: Ensuring patient-centered care. Retrieved from https://www.facs.org/~/media/files/quality%20programs/cancer/coc/progra…
Desch, C.E., Benson, A.B., Somerfield, M.R., Flynn, P. J., Krause, C., Loprinzi, C.L., . . . Petrelli, N.J. (2005). Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. Journal of Clinical Oncology, 23, 8512–8519. doi:10.1200/JCO.2005.04.0063
Dillman, D. (2007). Mail and Internet surveys: The tailored design method (2nd ed.). Hoboken, NJ: John Wiley and Sons.
Dulko, D., Pace, C.M., Dittus, K.L., Sprague, B.L., Pollack, L.A., Hawkins, N.A., & Geller, B.M. (2013). Barriers and facilitators to implementing cancer survivorship care plans. Oncology Nursing Forum, 40, 575–580. doi:10.1188/13.ONF.575-580
Fairley, T.L., Hawk, H., & Pierre, S. (2006). Health behaviors and quality of life of cancer survivors in Massachusetts, 2006: Data use for comprehensive cancer control. Preventing Chronic Disease, 7, 1–8.
Figueredo, A., Rumble, R.B., Maroun, J., Earle, C.C., Cummings, B., McLeod, R., . . . Zwaal, C. (2003). Follow-up of patients with curatively resected colorectal cancer: A practice guideline. BMC Cancer, 3, 26. doi:10.1186/1471-2407-3-26
Grant, M., Economou, D., & Ferrell, B.R. (2010). Oncology nurse participation in survivorship care. Clinical Journal of Oncology Nursing, 14, 709–715. doi:10.1188/10.CJON.709-715
Graubard, B.I., & Korn, E.L. (1999). Predictive margins with survey data. Biometrics, 55, 652–659. doi:10.1111/j.0006-341X.1999.00652.x
Guessous, I., Dash, C., Lapin, P., Doroshenk, M., Smith, R.A., & Klabunde, C.N. (2010). Colorectal cancer screening barriers and facilitators in older persons. Preventive Medicine, 50, 3–10. doi:10.1016/j.ypmed.2009.12.005
Hewitt, M., Greenfield, S., & Stovall, E. (2006). From cancer patient to cancer survivor: Lost in transition. Washington, DC: National Academies Press.
Howlader, N., Noone, A., Krapcho, M., Garshell, J., Neyman, N., Altekruse, S.F., . . . Cronin, K.A. (Eds.). (2013). SEER cancer statistics review: 1975–2010. Retrieved from http://seer.cancer.gov/csr/1975_2010/sections.html
Hudson, S.V., Hahn, K.A., Ohman-Strickland, P., Cunningham, R.S., Miller, S.M., & Crabtree, B.F. (2009). Breast, colorectal and prostate cancer screening for cancer survivors and non-cancer patients in community practices. Journal of General Internal Medicine, 24(Suppl. 2), S487–S490. doi:10.1007/s11606-009-1036-3
Jabson, J.M., & Bowen, D.J. (2013). Cancer treatment summaries and follow-up care instructions: Which cancer survivors receive them? Cancer Causes and Control, 24, 861–871.
Jeffery, M., Hickey, B.E., & Hider, P.N. (2007). Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database of Systematic Reviews, 1, CD002200. doi:10.1002/14651858.CD002200.pub2
Jones, R.M., Mongin, S.J., Lazovich, D., Church, T.R., & Yeazel, M.W. (2008). Validity of four self-reported colorectal cancer screening modalities in a general population: Differences over time and by intervention assignment. Cancer Epidemiology, Biomarkers and Prevention, 17, 777–784. doi:10.1158/1055-9965.EPI-07-0441
Khoja, S., McGregor, S.E., & Hilsden, R.J. (2007). Validation of self-reported history of colorectal cancer screening. Canadian Family Physician, 53, 1192–1197.
National Comprehensive Cancer Network. (2012). Clinical Practice Guidelines in Oncology: Colon cancer [v.3.2012]. Retrieved from http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
National Comprehensive Cancer Network & American Cancer Society. (2005). Colon and rectal cancer: Treatment guidelines for patients. Retrieved from http://www.colorectal-cancer.ca/IMG/pdf/NCCN-colorectal-guidelines.pdf
Partin, M.R., Grill, J., Noorbaloochi, S., Powell, A.A., Burgess, D.J., Vernon, S.W., . . . Fisher, D.A. (2008). Validation of self-reported colorectal cancer screening behavior from a mixed-mode survey of veterans. Cancer Epidemiology, Biomarkers and Prevention, 17, 768–776. doi:10.1158/1055-9965.EPI-07-0759
Rao, J., & Scott, A. (1987). On simple adjustments to chi-square tests with sample survey data. Annals of Statistics, 15, 385–397. doi:10.1214/aos/1176350273
Renehan, A.G., Egger, M., Saunders, M.P., & O’Dwyer, S.T. (2002). Impact on survival of intensive follow up after curative resection for colorectal cancer: Systematic review and meta-analysis of randomised trials. BMJ, 324, 813. doi:10.1136/bmj.324.7341.813
Rex, D.K., Kahi, C.J., Levin, B., Smith, R.A., Bond, J.H., Brooks, D., . . . Winawer, S.J. (2006). Guidelines for colonoscopy surveillance after cancer resection: A consensus update by the American Cancer Society and US Multi-Society Task Force on Colorectal Cancer. CA: A Cancer Journal for Clinicians, 56, 160–167. doi:10.1053/j.gastro.2006.03.013
Rolnick, S., Hensley Alford, S., Kucera, G.P., Fortman, K., Ulcickas Yood, M., Jankowski, M., & Johnson, C.C. (2005). Racial and age differences in colon examination surveillance following a diagnosis of colorectal cancer. Journal of the National Cancer Institute. Monographs, 35, 96–101. doi:10.1093/jncimonographs/lgi045
Rulyak, S.J., Mandelson, M.T., Brentnall, T.A., Rutter, C.M., & Wagner, E.H. (2004). Clinical and sociodemographic factors associated with colon surveillance among patients with a history of colorectal cancer. Gastrointestinal Endoscopy, 59, 239–247. doi:10.1016/S0016-5107(03)02531-8
Sabatino, S.A., Thompson, T.D., Smith, J.L., Rowland, J.H., Forsythe, L.P., Pollack, L., & Hawkins, N.A. (2013). Receipt of cancer treatment summaries and follow-up instructions among adult cancer survivors: Results from a national survey. Journal of Cancer Survivorship, 7, 32–43. doi:10.1007/s11764-012-0242-x
Subramanian, S., Klosterman, M., Amonkar, M.M., & Hunt, T.L. (2004). Adherence with colorectal cancer screening guidelines: A review. Preventive Medicine, 38, 536–550. doi:10.1016/j.ypmed.2003.12.011
Tjandra, J.J., & Chan, M.K. (2007). Follow-up after curative resection of colorectal cancer: A meta-analysis. Diseases of the Colon and Rectum, 50, 1783–1799. doi:10.1007/s10350-007-9030-5
Trask, P.C., Rabin, C., Rogers, M.L., Whiteley, J., Nash, J., Frierson, G., & Pinto, B. (2005). Cancer screening practices among cancer survivors. American Journal of Preventive Medicine, 28, 351–356.doi:10.1016/j.amepre.2005.01.005
U.S. Preventive Services Task Force. (2013). Published recommendations. Retrieved from http://www.uspreventiveservicestaskforce.org/BrowseRec/Index
Winawer, S., Fletcher, R., Rex D., Bond, J., Burt, R., Ferrucci, J., . . . Simmang, C. (2003). Colorectal cancer screening and surveillance: Clinical guidelines and rationale—Update based on new evidence. Gastroenterology, 124, 544–560. doi:10.1053/gast.2003.50044
About the Author(s)
Nikki A. Hawkins, PhD, is a behavioral scientist; Zahava Berk-owitz, MS, is a statistician; Juan L. Rodriguez, MPH, is an epidemiologist; and Jacqueline W. Miller, MD, Susan A. Sabatino, MD, MPH, and Lori A. Pollack, MD, MPH, are medical officers, all at the Centers for Disease Control and Prevention (CDC) in Atlanta, GA. This article was written in the course of employment by the United States Government and it is not subject to copyright in the United States. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. Data collection was undertaken through a CDC contract with ICF International (200-2002-00574). Hawkins can be reached at nhawkins@cdc.gov, with copy to editor at ONFEditor@ons.org. (Submitted September 2014. Accepted for publication December 17, 2014.)

