Effectiveness, Safety, and Tolerance of Scalp Cooling for Chemotherapy-Induced Alopecia
Problem Identification: There is a lack of guideline recommendations about the use of scalp cooling for preventing chemotherapy-induced alopecia (CIA). This overview was conducted to summarize effectiveness, safety, and tolerance of scalp cooling for CIA based on systematic reviews.
Literature Search: PubMed®, Embase®, Cochrane Library, and CNKI were searched from inception to May 15, 2021.
Data Evaluation: AMSTAR 2 was used to assess the methodologic quality. Qualitative and quantitative synthesis methods were used to identify the effectiveness, safety, and tolerance of scalp cooling.
Synthesis: 14 systematic reviews were identified, and the quality assessment was poor. Scalp cooling has been considered to be effective for preventing chemotherapy-induced alopecia and has been confirmed in patients with breast cancer and other solid tumors. Most adverse effects were mild and moderate, and scalp cooling did not increase the risk of scalp metastases.
Implications for Research: This overview could guide nurses to provide access to scalp cooling to reduce the risk of severe or total chemotherapy-induced alopecia for patients undergoing chemotherapy. The large-scale application of scalp cooling may be promoted by establishing reimbursement mechanisms and increasing available devices in the future.
Jump to a section
Chemotherapy-induced alopecia (CIA) is a reversible but common and highly distressing side effect that particularly refers to different levels of hair loss led by a single or combined chemotherapy regimen (Komen et al., 2013). In general, CIA starts one to three weeks after the first cycle of chemotherapy treatment and recovers within three to six months after chemotherapy ends (Oshima et al., 2001). Incidentally, some instances of permanent CIA could occur, likely because of high-dose combined drug therapy (Tosti et al., 2005; Trüeb, 2009). CIA inevitably occurs because about 85%–90% of scalp hair follicles are in the anagen phase at any given time (Koch et al., 2020), and just like malignant cells, they are sensitive to chemotherapy drugs because of increasing oxidation/reactive oxygen species levels and stimulating apoptosis of cells (Panieri & Santoro, 2016). Studies have shown that the incidence of CIA ranges from 10% to 100%, with rates from 10% to 50% for antimetabolites, greater than 60% for alkylating agents, greater than 80% for antimicrotubule agents, and from 60% to 100% for topoisomerase inhibitors (Roe, 2014; Trüeb, 2010). Although hair loss is a non–life-threatening condition, it causes negative body image and reminds people of cancer or other physiological suffering (Choi et al., 2014; van den Hurk et al., 2013). Patients experiencing CIA tend to have great psychological stress, such as anxiety, confusion, and depression, particularly for women and young men (Hilton et al., 2008). Meanwhile, CIA could affect self-esteem and social relationships. Women experiencing hair loss have expressed that it was more difficult to cope with than losing a breast (Chan et al., 2018). As a result, as many as 8% of patients refused to receive chemotherapy treatment or chose a less effective regimen to avoid severe alopecia (Hesketh et al., 2004).
In contrast to many other side effects of chemotherapy that have been treated by marked progress (including infection, pain, emesis, bone marrow suppression, and thrombosis) (Hesketh et al., 2004), progress made in preventing CIA has not been as significant. Among preventive measures for alopecia, scalp cooling is the most widely used, comparing with pharmacologic interventions such as minoxidil and other nonpharmacologic strategies such as scalp tourniquets (Shin et al., 2015). Scalp cooling therapy has been used since the 1970s, mainly to prevent and reduce the occurrence of CIA (Grevelman & Breed, 2005; Soref & Fahl, 2015). The initial method involved putting a plastic bag filled with crushed ice on the scalp fixed by bandages to lower the scalp temperature, and thereafter cold air, gel packs, or electronically cooled caps were gradually used in clinics (Guy et al., 1982; Pliskow et al., 2016). Currently, scalp cooling devices, such as the Paxman (United Kingdom) and Dignitana (Sweden) systems, are applied in more than 30 countries worldwide (Wang et al., 2021). They are available in several different sizes that can be suitable for the head, lower the temperature gradually, and may be more comfortable and well tolerated (Massey, 2004). In addition, a lot of literature, including primary studies and reviews, has reported the application of scalp cooling as an effective treatment for preventing CIA.
Unfortunately, the relevant recommendation on the use of scalp cooling for CIA was not found in any clinical practice guidelines, possibly because of insufficient data from existing trials to make recommendations on interventions to prevent or treat side effects and symptoms related to alopecia (Greenlee et al., 2014). In addition, the authors found that there was still some ambiguous information about scalp cooling, such as treatment time, targeted temperature, and weaknesses of the implementation. Therefore, in consideration of various available descriptions about scalp cooling therapy’s effectiveness, safety, and tolerance, the authors integrated the systematic reviews, which were considered as high-level evidence, in this overview to combine information and make a comprehensive evaluation accessible to people experiencing CIA.
The aim of this article is to conduct an overview of systematic reviews to identify and summarize evidence of the effect evaluation of scalp cooling for preventing CIA.
Methods
This overview of systematic reviews was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines and the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Page et al., 2021).
The included studies were assessed for eligibility based on the inclusion and exclusion criteria, evaluating for types of study designs, participants, interventions, and outcomes.
Systematic reviews published in peer-reviewed journals were included. Therefore, the narrative reviews and reviews that did not follow the methodology of a systematic review were excluded (Pae, 2015). Conference abstracts, editorials, unpublished work, and other unavailable full-text studies were also excluded. The publication language was limited to Chinese and English, and there were no restrictions to the publication date.
Regardless of age, gender, and ethnicity, patients diagnosed with cancer and treated with chemotherapy or adjuvant chemotherapy were considered in the current study. In addition, any restriction to types of cancer and chemotherapy drugs was not applied.
Studies were included if they used scalp cooling for preventing CIA. Studies could involve any types of scalp hypothermia devices and technologies, but not in combination with other CIA-preventive interventions.
Studies were included if they examined at least one measure of the efficacy of scalp cooling, such as the occurrence, extent, or severity of CIA; a series of adverse events; the longer-term sequelae; and any signs of intolerance.
Search Strategy and Selection
A comprehensive search was performed via four electronic databases, including PubMed®, Embase®, Cochrane Library, and CNKI from their inception to May 15, 2021, using MeSH (Medical Subject Headings) terms and free words. An additional manual search was performed via screening reference lists of included systematic reviews.
Two reviewers (X.-Y.Z. and K.-L.Y.) selected the records from the databases independently by screening titles and abstracts. Full-text articles were acquired from all studies identified as potentially relevant after the consent of both reviewers, and were assessed independently in accordance with the inclusion and exclusion criteria. Any disagreement between the two reviewers was resolved by discussion until consensus was achieved.
Data Extraction, Assessment, and Synthesis
At the outset, data pre-extraction was performed through two to three studies included in the authors’ search, and the extraction form has been modified by all researchers. Then, two reviewers (X.-Y.Z. and K.-L.Y.) extracted the data from all included systematic reviews independently. The following information was extracted: the first author, year of publication, type and number of included studies, inclusion and exclusion criteria, patients, type of cancer, chemotherapy regimen, implementation details of scalp cooling and control methods, assessment criteria of CIA, clinical outcomes, adverse events, and so on.
The methodologic quality of identified systematic reviews was assessed independently by two reviewers (X.-Y.Z. and K.-L.Y.) using the AMSTAR (Assessment of the Methodological Quality of Systematic Reviews) 2 tool, which has shown good inter-rater reliability and construct validity (Shea et al., 2017). The AMSTAR 2 tool consists of 16 items evaluating relevant methodologic aspects and includes 7 critical items (items 2, 4, 7, 9, 11, 13, and 15). Each item was rated as yes (totally done), partial yes (partially done), no (clearly not done), or not applicable. Instead of generating an overall score, AMSTAR 2 judged systematic reviews as high (one noncritical item or less rated as no), moderate (more than one noncritical item rated as no), low (one critical item or less rated as no), and critically low (more than one critical item rated as no) by interpreting weaknesses detected in critical and noncritical items.
The percentage and bar graph were used to show the results of the AMSTAR 2. The quantitative results that estimated the effectiveness of scalp cooling were presented as a forest plot. In addition to a quantitative analysis, a qualitative synthesis of included systematic reviews was also performed because of the different design types. To explore more details of scalp cooling treatment, the strengths and weaknesses of interventions and limitations of systematic reviews were summarized.
Results
The PRISMA flow diagram of the literature search and study selection is shown in Figure 1. A total of 580 potentially relevant reports were initially identified, and 14 systematic reviews were included.
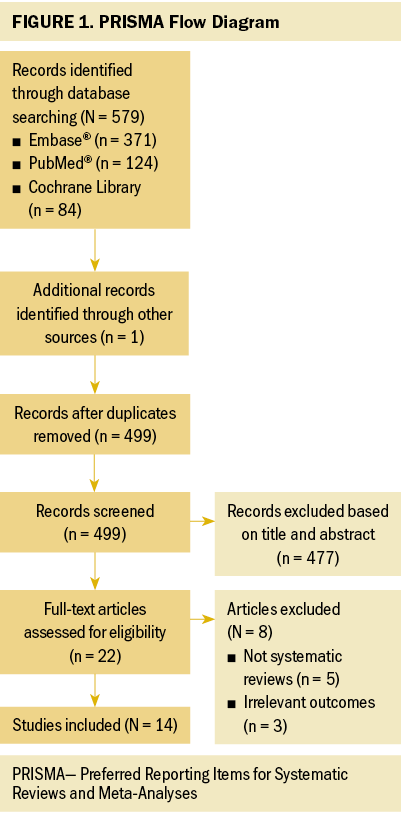
General Characteristics of Included Studies
The general characteristics of included systematic reviews are summarized in Table 1. All systematic reviews were published in English, and the year of publications ranged from 2008 to 2021. Ten systematic reviews evaluated the efficacy of scalp cooling for prevention of CIA (Ding et al., 2020; Haque et al., 2020; Lotfi-Jam et al., 2008; Marks et al., 2018; Ross & Fischer-Cartlidge, 2017; Rugo & Voigt, 2018; Shah et al., 2018; Shin et al., 2015; Wang et al., 2021; Zhou et al., 2020); two systematic reviews reported pathogenesis of CIA (Rubio-Gonzalez et al., 2018) and influencing factors of effectiveness of scalp cooling (Komen et al., 2013); one systematic review evaluated the effect of scalp cooling on quality of life of those experiencing CIA (Marks et al., 2019); and one systematic review showed the risk of scalp metastases with scalp cooling for CIA (Rugo et al., 2017). The largest proportion of the included participants was individuals with breast cancer. The types of scalp cooling technologies were cooling caps (including Gel cap, Chemocap, Penguin, and Spenco) and scalp cooling systems (including Paxman and DigniCap®). The primary outcome of included systematic reviews was effectiveness of scalp cooling described by the extent of alopecia.
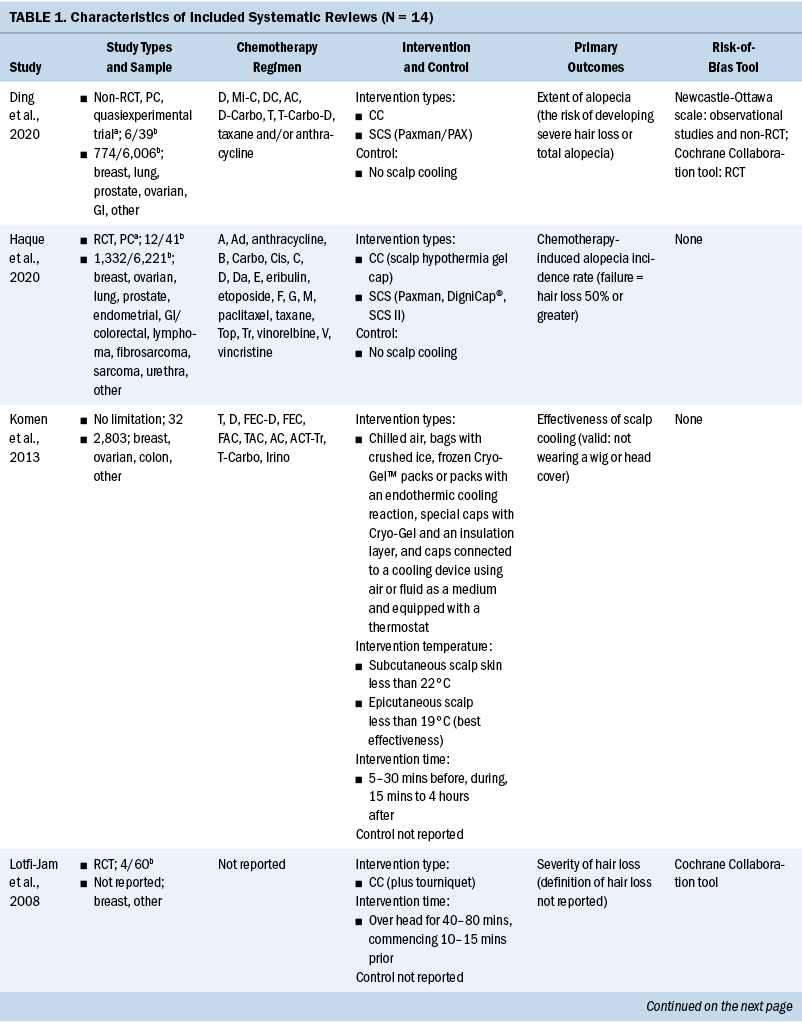
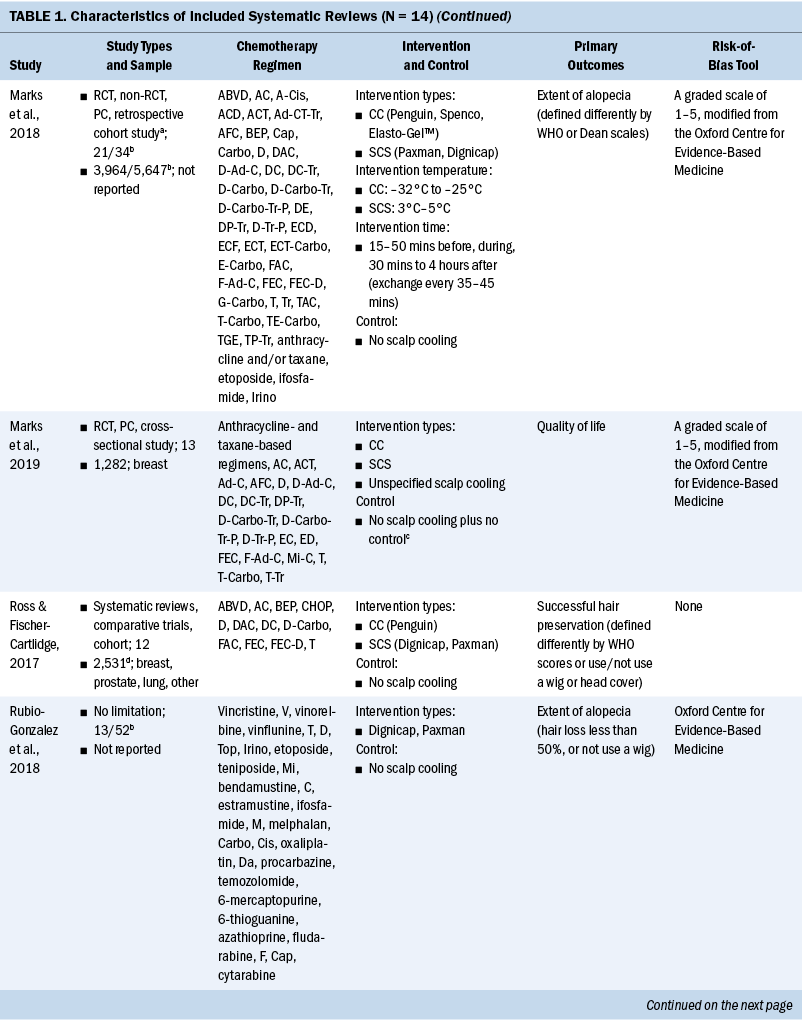
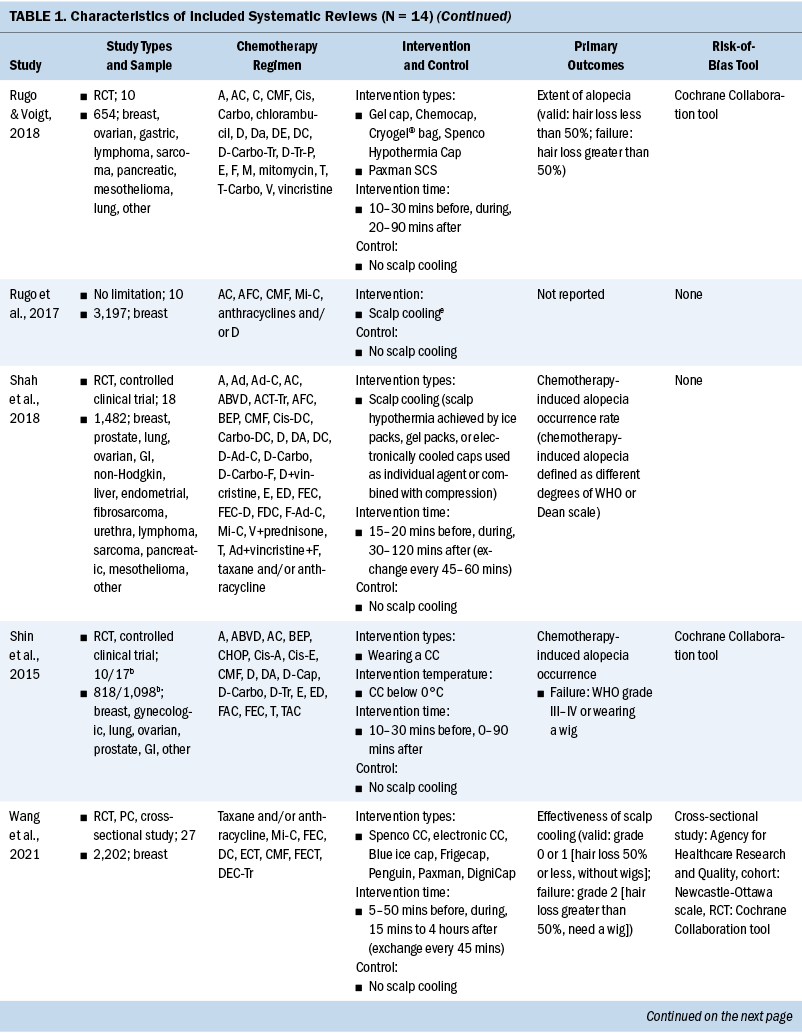
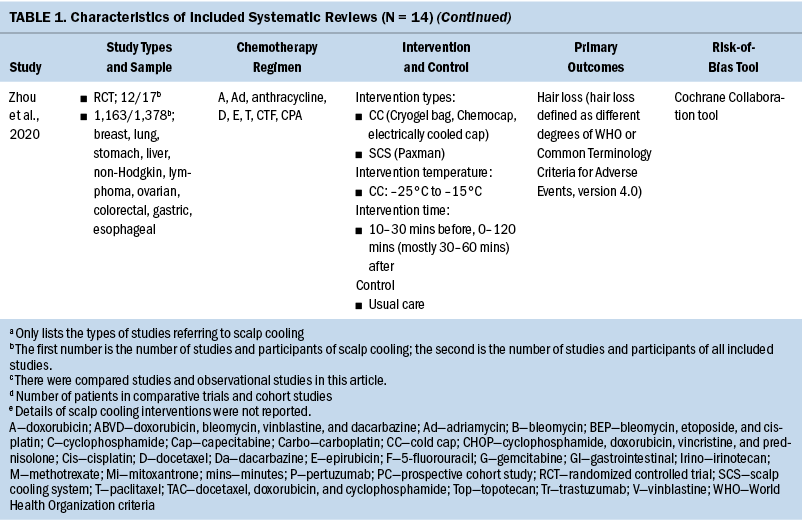
Quality Assessment of Included Studies
Among 14 systematic reviews, 3 were rated as low-quality (Rugo & Voigt, 2018; Shin et al., 2015; Wang et al., 2021) and 11 were rated as critically low-quality (Ding et al., 2020; Haque et al., 2020; Komen et al., 2013; Lotfi-Jam et al., 2008; Marks et al., 2018, 2019; Ross & Fischer-Cartlidge, 2017; Rubio-Gonzalez et al., 2018; Rugo et al., 2017; Shah et al., 2018; Zhou et al., 2020). Four items (items 1 [100%], 4 [100%], 8 [71.4%], and 16 [78.6%]) were reported at more than 70% adherence, and seven items (items 2, 3, 6, 7, 10, 13, and 15) were reported at below 30% adherence.
Effect Evaluation of Scalp Cooling
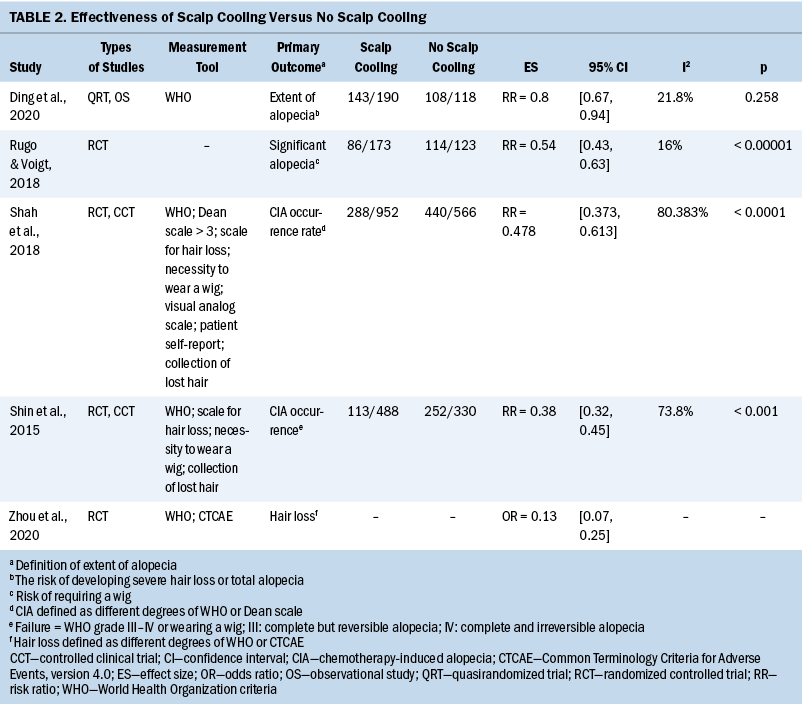
Effectiveness: All included systematic reviews demonstrated that scalp cooling was the most common and effective method among preventive interventions for CIA. It was found that the use of scalp cooling significantly reduced the risk of severe or total alopecia from five systematic reviews that conducted meta-analyses (Ding et al., 2020; Rugo & Voigt, 2018; Shah et al., 2018; Shin et al., 2015; Zhou et al., 2020). The results are listed in Table 2.
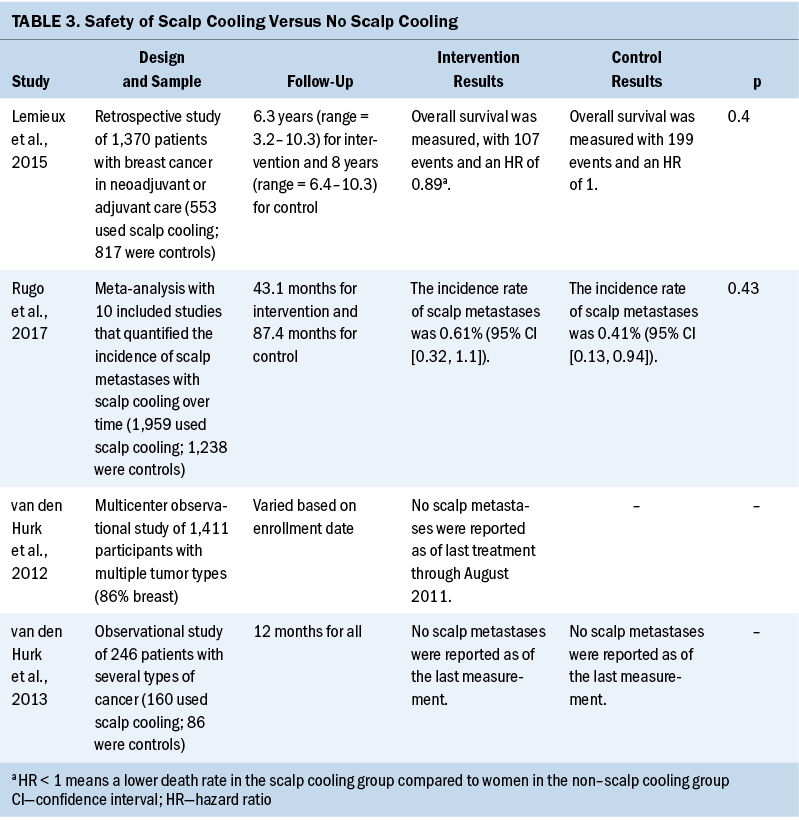
Safety: Among the included 14 systematic reviews, only 1 systematic review took the incidence rate of scalp metastases from scalp cooling for CIA as the primary outcome and performed quantitative synthesis (Rugo et al., 2017). Beyond that, three more included studies (Lemieux et al., 2015; van den Hurk et al., 2012, 2013) relevant to scalp metastases and overall survival in two other systematic reviews (Ding et al., 2020; Ross & Fischer-Cartlidge, 2017) were also found. Safety data are shown in Table 3 and suggested that scalp cooling was unlikely to adversely affect incidence of scalp metastases and overall survival.
Tolerance: Overall, scalp cooling has good tolerance. Included systematic reviews reported secondary outcomes, including quality of life (QOL), body image perception, depression and anxiety, and adverse effects. Zhou et al. (2020) indicated that the changes in social function, emotional function, and body image perception were not different between the scalp cooling and no scalp cooling groups. Marks et al. (2019) evaluated the relationship between use of scalp cooling and QOL in individuals with CIA and demonstrated that scalp cooling was not consistently associated with significant QOL improvements as assessed by the European Organisation for Research and Treatment of Cancer QOL Questionnaire—Core and Breast Cancer Module. Most adverse effects that were reported in studies were mild and moderate, including cold sensation, chills, dizziness, headache, scalp pain, head discomfort, the weight of the cap, and claustrophobia (Haque et al., 2020; Komen et al., 2013; Marks et al., 2018; Ross & Fischer-Cartlidge, 2017; Rugo et al., 2017; Rugo & Voigt, 2018; Wang et al., 2021; Zhou et al., 2020). Among them, the most common were cold and headache, with an incidence of about 4%–33.3% (Shin et al., 2015).
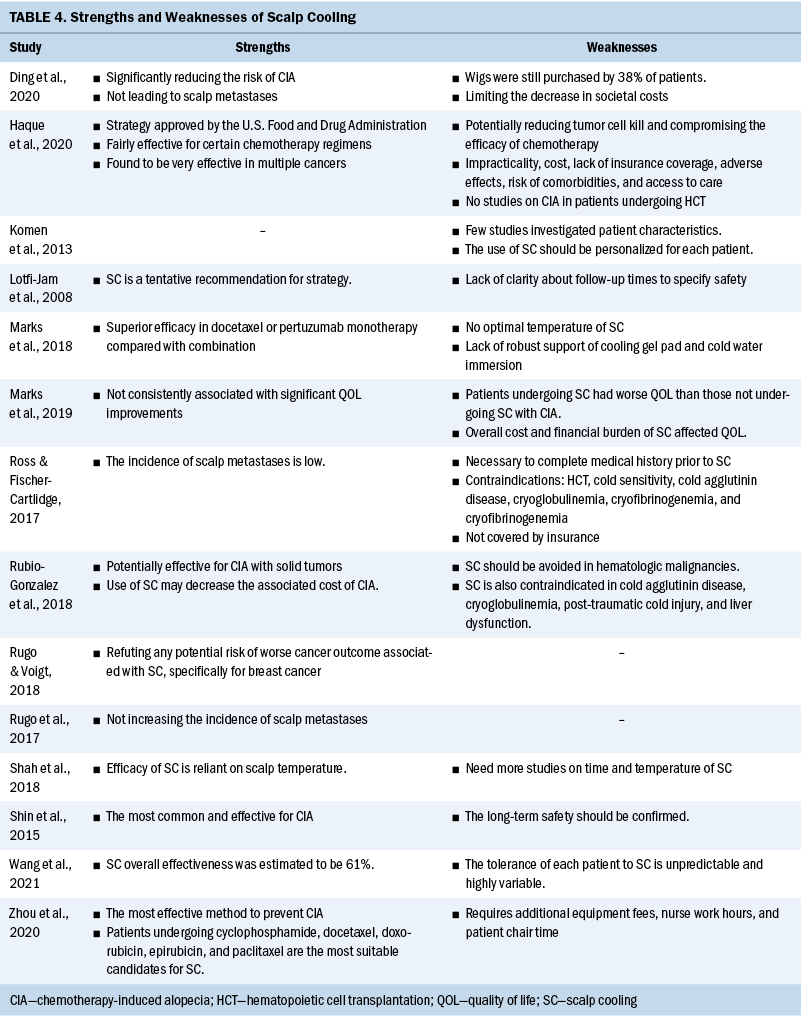
Strengths and weaknesses: Aside from the effect evaluation of scalp cooling, the strengths and weaknesses of this intervention are also shown in Table 4. As the only preventive intervention for CIA that was approved by the U.S. Food and Drug Administration (Haque et al., 2020; Rubio-Gonzalez et al., 2018), scalp cooling played a positive role and was used more widely. Because most systematic reviews were aimed at patients with solid tumors, scalp cooling was particularly recommended for these patients, and the corresponding commonly used chemotherapy regimens were anthracyclines, cyclophosphamides, and taxanes (Rugo & Voigt, 2018). Because of lack of safety data, scalp cooling should be avoided for patients with certain diseases, such as hematologic malignancies, and a complete medical history should be performed.
Discussion
This article identified 14 systematic reviews assessing the effect evaluation of scalp cooling treatment for preventing CIA. Three systematic reviews were rated as low-quality, and 11 were rated as critically low-quality. Scalp cooling was found to significantly reduce the risk of severe or total alopecia for patients receiving chemotherapy and had good safety and tolerance in clinical practice.
Hair is connected with general well-being, sexual attraction, strong social status, and other meanings beyond itself in modern life (Can et al., 2013). People with hair loss experience negative emotions in daily life, such as depression, anxiety, low self-esteem, and loss of sexuality. Some women have reported feeling that their personal identity would disappear along with the loss of hair and that lack of self-esteem could continue after the chemotherapy has finished and hair has regrown (Kim et al., 2012; Roe, 2014). Women tend to be constantly concerned about whether they could have new hair growth, and it is possible that there will be no regrowth (Kim et al., 2012). If they wear a wig or hat, besides any discomfort such as feeling hot and sweaty, they may become fearful about the wig blowing off in front of others (Roe, 2014). Even if new hair grows, patients may find that they have to accept the new hair with a different color because chemotherapy strips protein and affects the pigmentary unit of the hair follicle (Paus et al., 2013). Scalp cooling was reported as effective for preventing CIA. By lowering the scalp temperature, scalp cooling can cause rapid vasoconstriction and greatly reduce blood flow and chemotherapy infusion in the scalp area (Bülow et al., 1985; Janssen et al., 2007). Simultaneously, the rate of drug diffusion and metabolism of hair follicle cells are decreased at a low temperature (Lane et al., 1987). Consequently, the chemotherapeutic drugs are less harmful to the hair follicle cells in the scalp and the extent of CIA is reduced.
Scalp cooling has been commonly used to prevent CIA. Throughout the existing studies, patients with breast cancer were the largest number of participants. This may be related to the chemotherapy regimens for early-stage breast cancer, anthracycline and taxane–based therapies, which have been proven to be commonly associated with complete or severe alopecia by inducing hair follicle apoptosis at a greater frequency and severity than most other drugs (Simon et al., 2000). Meanwhile, female patients focus more on their appearance than male patients. Therefore, individuals with breast cancer have become the group with the largest demand for scalp cooling to prevent CIA. At present, increasing evidence shows that scalp cooling could relieve the severity of hair loss compared to no scalp cooling or usual care. This article represents a synthetic and complete review of high-level evidence from included systematic reviews on the effectiveness, safety, and tolerance of scalp cooling. Owing to the limited number of present randomized controlled trials in this field, the authors did not restrict the types of included studies in this article.
Quality Assessment
According to the methodologic quality assessment of AMSTAR 2 (Shea et al., 2017), 3 studies were rated as low-quality and the remaining 11 were rated as critically low-quality. The weaknesses of critical items were mainly cited as lack of review methods prior to the review, not providing a list of excluded studies and the reasons for exclusions, not accounting for risk of bias in individual studies, and not carrying out an adequate investigation of publication bias. Lack of protocol (item 2) may cause a larger adjustment or change during the study process, which could influence the rigor and precision of the systematic reviews. Therefore, prospective registration tends to be encouraged to avoid the risk of bias in methodology and facilitate processing transparency (Stewart et al., 2012). Lack of a list of excluded studies and the reasons for exclusions (item 7) would likely affect the authenticity of the results and increase selection bias. In a high-quality systematic review, it is an indispensable and necessary part to list the potentially relevant studies that do not meet the inclusion criteria and account the reason for exclusion. Lack of account for risk of bias in individual studies (item 13) and lack of assessment of publication bias (item 15) may destroy the authenticity of the conclusion (Shea et al., 2007). High-quality systematic reviews and meta-analyses can provide strong evidence and promote clinical practice, so researchers should abide by the rules of the relevant items of the AMSTAR 2 and entirely manage the methodologic quality of the studies.
Among studies in this field, there have not been many well-designed randomized controlled trials and controlled clinical trials. Observational and retrospective studies were in the majority, and this could potentially be linked to the characteristics and conditions of participants. The quality of most included studies was found to be poor. The risk of selective reporting was high because almost all studies were not preregistered. In regard to the heterogeneity, the types of cancers and chemotherapy regimens were various. The cooling time, frequency, and temperature of scalp cooling treatment differed and were not unified. The measurement tools, assessment time, and follow-up time were also inconsistent (Ding et al., 2020; Zhou et al., 2020). All these factors may decrease the overall methodologic quality of the studies. Therefore, there is an urgent need for well-designed randomized controlled trials to test the long-term effectiveness of scalp cooling and the management of adverse effects (Lotfi-Jam et al., 2008).
Effect Evaluation of Scalp Cooling
Influence factors: Scalp cooling has made great progress in efficacy and technology since the 1970s (Dunnill et al., 2018). Among all preventive interventions for CIA, the scalp cooling system has been approved by the U.S. Food and Drug Administration. However, the effectiveness of scalp cooling was not always satisfactory and it depended on several factors, including chemotherapy regimen, scalp temperature, cooling time, and patient characteristics. In general, scalp cooling was less favorable for patients undergoing combination drug therapy or higher doses compared to monotherapy or lower doses. In a Dutch observational study that collected data on effectiveness of scalp cooling for the past four years (from 2006 to 2009), for patients treated with different doses of 5-fluorouracil, epirubicin, and cyclophosphamide (FEC), 52% of patients treated with FEC (500, 90, and 500 mg/m2, respectively) did not require a head cover, versus 33% of patients treated with FEC (500, 100, and 500 mg/m2, respectively). For patients treated with docetaxel, 79% of patients treated with 75 mg/m2 did not require a head cover, versus 59% of patients treated with 100 mg/m2 (van den Hurk et al., 2012). Komen et al. (2013) indicated that scalp cooling failed to prevent hair loss in most patients who were treated with the combination of docetaxel, adriamycin, and cyclophosphamide chemotherapy for early-stage breast cancer. Some drugs, such as doxorubicin, were proven to be likely to enter cells via active transport mechanisms, which would be reduced by cooling. In addition, the cell models showed that doxorubicin-induced damage to DNA was reduced at lower temperatures (Lane et al., 1987). Likewise, Shin et al. (2015) reported that scalp cooling should be the recommended preventive intervention for CIA in individuals with breast cancer undergoing doxorubicin-, epirubicin-, or docetaxel-containing chemotherapy regimens.
Scalp cooling could decrease the drug dose and metabolism of the hair follicle by virtue of cooling the scalp. In existing studies, cooling temperature represented an essential variable, and ability to maintain the proper temperature during the treatment had a large effect on the success of scalp cooling (Katsimbri et al., 2000). However, no optimal temperature has been determined to prevent CIA. Zhou et al. (2020) reported cooling caps were previously cooled to –25°C to –15°C. Marks et al. (2018) showed that cooling caps were frozen from –32°C to –25°C, and the scalp cooling system could maintain from 3°C to 5°C. Shin et al. (2015) suggested the cooling cap was fully refrigerated below 0°C. To ensure prevention of hair loss, the epicutaneous scalp should be cooled to less than 19°C, and the subcutaneous scalp skin temperature should reach less than 22°C consistently (Bülow et al., 1985). Likewise, there was no clarified cooling time, which ranged from 5–50 minutes (mostly 15–30 minutes) prior to and during chemotherapy, and from 15 minutes to 4 hours (mostly 30–60 minutes) after chemotherapy. Komen et al. (2013) suggested 45 minutes as an optimal preinfusion cooling time because scalp temperature could reach a constant level of about 18°C (an ideal temperature) after 45 minutes. In addition, the tolerance of each patient to scalp cooling is highly variable and must be considered. Cold sensation and intolerance of hypothermia can lead to early cessation of scalp cooling, with requirement for removal (Shah et al., 2018). Therefore, the implementation of scalp cooling should be personalized for each patient by keeping a balance between patients’ tolerance and the threshold level of scalp temperature (Komen et al., 2013). In addition, Wang et al. (2021) believed that studies in the future should focus more on how to improve patients’ tolerance so they could experience chemotherapy more comfortably.
Patient characteristics are also an influencing factor for the effect of scalp cooling, specifically referring to the fact that scalp cooling was more effective for younger, male, and White individuals compared to older, female, and African American individuals (Komen et al., 2013). Because of aging skin and declining organ function, older adults’ scalp is likely to diminish the cold-induced vasoconstriction effect, which may cause a greater concentration of chemotherapy in the hair root cells (van den Hurk et al., 2012). Wang et al. (2021) concluded that scalp cooling therapy’s effectiveness rates were 67% (95% confidence interval [CI] [57, 62]) in European patients versus 53% (95% CI [44, 62) in Asian patients. This may be related to the fact that scalp cooling technology is more mature in European areas. Medical personnel in Europe may know more about the importance of scalp cooling and could provide better cooling service for patients, such as appropriate cooling time and temperature control. With more scalp cooling equipment, patients receiving chemotherapy are more likely to receive the scalp cooling intervention treatment in Europe. Janssen et al. (2005) proposed that the thickness of the hair layer could affect the effectiveness of scalp cooling, which may explain the poor effect of scalp cooling in African American individuals. A thicker hair layer acts as an insulating layer between the scalp and cooling cap, and prevents the scalp from being fully cooled.
Adverse effects: Most adverse effects from scalp cooling were reported to be mild and moderate, such as being cold and experiencing a headache. Although most participants said that scalp cooling was well tolerated, the previously mentioned side effects should be paid more attention, particularly chills, head discomfort, and claustrophobia, which may be emotional factors influencing the practice. Nurses should focus on the feelings of patients undergoing scalp cooling and help to relieve the discomfort with change in positioning, prophylactic painkillers, and additional blankets or pillows (Shah et al., 2018). Few patients required removal because of intolerability of the cold cap (Rugo & Voigt, 2018).
Marks et al. (2019) demonstrated that scalp cooling is not consistently associated with significant QOL improvements. In addition, some patients undergoing scalp cooling were reported to have worse QOL outcomes compared to those who did not undergo scalp cooling (van den Hurk et al., 2010). It is likely related to the uncertainty of hair loss and overall disappointment in unsuccessful scalp cooling. Patients undergoing cooling treatment possibly placed a higher importance on hair preservation and focused more on occurrence of hair loss.
Application analysis: At present, there have been almost no studies on scalp cooling preventing CIA in patients with hematologic malignancies. This may be related to concerns about tumor metastases, failed chemotherapy, or even reduced survival rates. However, the chemotherapy doses for a patient with a hematologic malignancy are typically 5–10 times higher than those given for solid tumors, and permanent CIA occurs in some patients with hematologic malignancies. Therefore, alopecia in these patients should be paid more attention. Because there has been a lack of data, scalp cooling should be prohibited in patients with hematologic malignancies, as well as those with cold sensitivity, cold agglutinin disease, cryoglobulinemia, and cryofibrinogenemia (Komen et al., 2013; Rubio-Gonzalez et al., 2018). In addition, patients with abnormal liver function were not suggested to receive scalp cooling therapy because liver function is associated with metabolism of chemotherapeutics (Lemenager et al., 1997). Therefore, prior to recommending scalp cooling, a complete medical history should be performed, and the long-term safety of scalp cooling should be evaluated in future studies (Ross & Fischer-Cartlidge, 2017).
Depending on the specific cooling device and geographic location, it is inevitable that scalp cooling therapy will increase cost during the chemotherapy period. Nangia et al. (2017) reported that scalp cooling can cost roughly $1,500–$3,000 per patient in the United States, on average. Currently, scalp cooling is not covered by health insurance (Nangia et al., 2017). In addition, the large-scale application of scalp cooling devices requires additional equipment fees, nursing care hours, and patient chair time (Kruse & Abraham, 2018). The chemotherapy infusion centers may be promoted by increasing available cooling devices and extending cooling time. Therefore, before transferring theory to large-scale practice, these factors need to be considered.
Limitations
There were several limitations in this article. First, only English and Chinese studies were searched, and only English systematic reviews were included, so a small number of studies in other languages were likely missed. Second, because there was not a unified definition of the success of hair preservation after scalp cooling treatment, the authors did not perform quantitative analysis. Last, quality assessment of included studies was low or critically low, and this may be likely to affect the credibility of the conclusions.
Implications for Nursing
As a common but non–life-threatening side effect, CIA was paid less attention than other chemotherapeutic adverse effects, such as infection, pain, emesis, and bone marrow suppression. However, CIA causes discomfort and psychological burden for patients undergoing chemotherapy. It adds to life difficulties after cancer survival, and this should not be ignored. Scalp cooling has been used widely for preventing CIA, but there was a lack of relevant recommendation in clinical guidelines. This overview integrated the existing systematic reviews about the use of scalp cooling for CIA and showed that scalp cooling was significantly effective for preventing CIA, particularly for patients with breast cancer and other solid tumors, because of its safety and tolerance. In addition, some ambiguous information about scalp cooling was presented, such as treatment time, targeted temperature, and the weaknesses of the implementation. This evidence could guide nurses to provide access to scalp cooling to reduce the risk of severe or total alopecia for patients undergoing chemotherapy and fit the treatment to patients’ context and goals. Future clinical practice can move the evidence base forward by conducting well-designed research and resolving problems with cost and devices.
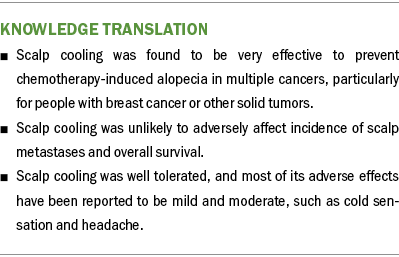
Conclusion
In the current overview, all studies reported on the use of scalp cooling intervention for CIA, including its effectiveness, safety, tolerance, strengths, and weaknesses. Overall, scalp cooling was found to be very effective to prevent CIA in multiple cancers, particularly for those with breast cancer or other solid tumors. However, because of the high heterogeneity of included participants and interventions, no unified measurement standard, no clarified cooling time, and the poor quality assessment of included studies, there is an urgent need for well-designed randomized controlled trials to perform normative interventions and to test the long-term effectiveness of scalp cooling for the management of hair loss caused by chemotherapy.
About the Authors
Xin-Yu Zhang, MN, is a secretary in the Nursing Department at Chengdu BOE Hospital in Sichuan Province, China; Ke-Lu Yang, MN, is a doctoral student in the Department of Public Health and Primary Care in the Academic Centre for Nursing and Midwifery at KU Leuven-University in Leuven, Belgium; Wen-Qing Liu, BSN, is the director of the Nursing Department, and Jie Huang, BSN, is a head nurse and clinical nurse specialist in the Oncology Department, both at Chengdu BOE Hospital; and Ning Ning, BSN, is a professor and doctoral supervisor in the West China School of Nursing at Sichuan University in Chengdu, China. This work was supported by Chengdu BOE Hospital. The content is solely the responsibility of the authors and does not necessarily represent the official views of the hospital. Zhang, Yang, and Huang contributed to the conceptualization and design. All authors completed the data collection. Zhang and Yang provided analysis and statistical support. Zhang, Liu, Huang, and Ning contributed to the manuscript preparation. Zhang can be reached at 10159558@boe.com.cn, with copy to ONFEditor@ons.org. (Submitted August 2021. Accepted December 20, 2021.)
References
Bülow, J., Friberg, L., Gaardsting, O., & Hansen, M. (1985). Frontal subcutaneous blood flow, and epi-and subcutaneous temperatures during scalp cooling in normal man. Scandinavian Journal of Clinical and Laboratory Investigation, 45(6), 505–508. https://doi.org/10.3109/00365518509155250
Can, G., Demir, M., Erol, O., & Aydiner, A. (2013). A comparison of men and women’s experiences of chemotherapy-induced alopecia. European Journal of Oncology Nursing, 17(3), 255–260. https://doi.org/10.1016/j.ejon.2012.06.003
Chan, A., Bauwens, A., Pontre, S., Jackson, S., McGlone, F., Ernenwein, T., . . . Reid, C. (2018). Efficacy of scalp cooling in reducing alopecia in early breast cancer patients receiving contemporary chemotherapy regimens. Breast, 41, 127–132. https://doi.org/10.1016/j.breast.2018.07.006
Choi, E.K., Kim, I.R., Chang, O., Kang, D., Nam, S.J., Lee, J.E., . . . Cho, J. (2014). Impact of chemotherapy-induced alopecia distress on body image, psychosocial well-being, and depression in breast cancer patients. Psycho-Oncology, 23(10), 1103–1110. https://doi.org/10.1002/pon.3531
Ding, J., Farah, M.H., Nayfeh, T., Malandris, K., Manolopoulos, A., Ginex, P.K., . . . Murad, M.H. (2020). Targeted therapy– and chemotherapy-associated skin toxicities: Systematic review and meta-analysis. Oncology Nursing Forum, 47(5), E149–E160. https://doi.org/10.1188/20.ONF.E149-E160
Dunnill, C.J., Al-Tameemi, W., Collett, A., Haslam, I.S., & Georgopoulos, N.T. (2018). A clinical and biological guide for understanding chemotherapy-induced alopecia and its prevention. Oncologist, 23(1), 84–96. https://doi.org/10.1634/theoncologist.2017-0263
Greenlee, H., Balneaves, L.G., Carlson, L.E., Cohen, M., Deng, G., Hershman, D., . . . Tripathy, D. (2014). Clinical practice guidelines on the use of integrative therapies as supportive care in patients treated for breast cancer. JNCI Monographs, 2014(50), 346–358. https://doi.org/10.1093/jncimonographs/lgu041
Grevelman, E.G., & Breed, W.P.M. (2005). Prevention of chemotherapy-induced hair loss by scalp cooling. Annals of Oncology, 16(3), 352–358. https://doi.org/10.1093/annonc/mdi088
Guy, R., Parker, H., Shah, S., & Geddes, D. (1982). Scalp cooling by thermocirculator. Lancet, 319(8278), 937–938. https://doi.org/10.1016/s0140-6736(82)91936-5
Haque, E., Alabdaljabar, M.S., Ruddy, K.J., Haddad, T.C., Thompson, C.A., Lehman, J.S., & Hashmi, S.K. (2020). Management of chemotherapy-induced alopecia (CIA): A comprehensive review and future directions. Critical Reviews in Oncology/Hematology, 156, 103093. https://doi.org/10.1016/j.critrevonc.2020.103093
Hesketh, P.J., Batchelor, D., Golant, M., Lyman, G.H., Rhodes, N., & Yardley, D. (2004). Chemotherapy-induced alopecia: Psychosocial impact and therapeutic approaches. Supportive Care in Cancer, 12(8), 543–549. https://doi.org/10.1007/s00520-003-0562-5
Hilton, S., Hunt, K., Emslie, C., Salinas, M., & Ziebland, S. (2008). Have men been overlooked? A comparison of young men and women’s experiences of chemotherapy-induced alopecia. Psycho-Oncology, 17(6), 577–583. https://doi.org/10.1002/pon.1272
Janssen, F.E.M., Van Leeuwen, G.M.J., & Van Steenhoven, A.A. (2005). Modelling of temperature and perfusion during scalp cooling. Physics in Medicine and Biology, 50(17), 4065–4073. https://doi.org/10.1088/0031-9155/50/17/010
Janssen, F.P.E.M., Rajan, V., Steenbergen, W., van Leeuwen, G.M.J., & van Steenhoven, A.A. (2007). The relationship between local scalp skin temperature and cutaneous perfusion during scalp cooling. Physiological Measurement, 28(8), 829–839. https://doi.org/10.1088/0967-3334/28/8/006
Katsimbri, P., Bamias, A., & Pavlidis, N. (2000). Prevention of chemotherapy-induced alopecia using an effective scalp cooling system. European Journal of Cancer, 36(6), 766–771. https://doi.org/10.1016/s0959-8049(00)00012-5
Kim, I.R., Cho, J.H., Choi, E.K., Kwon, I.G., Sung, Y.H., Lee, J.E., . . . Yang, J.H. (2012). Perception, attitudes, preparedness and experience of chemotherapy-induced alopecia among breast cancer patients: A qualitative study. Asian Pacific Journal of Cancer Prevention, 13(4), 1383–1388. https://doi.org/10.7314/apjcp.2012.13.4.1383
Koch, S.L., Tridico, S.R., Bernard, B.A., Shriver, M.D., & Jablonski, N.G. (2020). The biology of human hair: A multidisciplinary review. American Journal of Human Biology, 32(2), e23316. https://doi.org/10.1002/ajhb.23316
Komen, M.M.C., Smorenburg, C.H., van den Hurk, C.J.G., & Nortier, J.W.R. (2013). Factors influencing the effectiveness of scalp cooling in the prevention of chemotherapy-induced alopecia. Oncologist, 18(7), 885–891. https://doi.org/10.1634/theoncologist.2012-0332
Kruse, M., & Abraham, J. (2018). Management of chemotherapy-induced alopecia with scalp cooling. Journal of Oncology Practice, 14(3), 149–154. https://doi.org/10.1200/JOP.17.00038
Lane, P., Vichi, P., Bain, D.L., & Tritton, T.R. (1987). Temperature dependence studies of adriamycin uptake and cytotoxicity. Cancer Research, 47(15), 4038–4042.
Lemenager, M., Lecomte, S., Bonneterre, M.E., Bessa, E., Dauba, J., & Bonneterre, J. (1997). Effectiveness of cold cap in the prevention of docetaxel-induced alopecia. European Journal of Cancer, 33(2), 297–300. https://doi.org/10.1016/s0959-8049(96)00374-7
Lemieux, J., Provencher, L., Perron, L., Brisson, J., Amireault, C., Blanchette, C., & Maunsell, E. (2015). No effect of scalp cooling on survival among women with breast cancer. Breast Cancer Research and Treatment, 149(1), 263–268. https://doi.org/10.1007/s10549-014-3231-0
Lotfi-Jam, K., Carey, M., Jefford, M., Schofield, P., Charleson, C., & Aranda, S. (2008). Nonpharmacologic strategies for managing common chemotherapy adverse effects: A systematic review. Journal of Clinical Oncology, 26(34), 5618–5629. https://doi.org/10.1200/JCO.2007.15.9053
Marks, D.H., Okhovat, J.P., Hagigeorges, D., Manatis-Lornell, A.J., Isakoff, S.J., Lacouture, M.E., & Senna, M.M. (2019). The effect of scalp cooling on CIA-related quality of life in breast cancer patients: A systematic review. Breast Cancer Research and Treatment, 175(2), 267–276. https://doi.org/10.1007/s10549-019-05169-0
Marks, D.H., Qureshi, A., & Friedman, A. (2018). Evaluation of prevention interventions for taxane-induced dermatologic adverse events: A systematic review. JAMA Dermatology, 154(12), 1465–1472. https://doi.org/10.1001/jamadermatol.2018.3465
Massey, C.S. (2004). A multicentre study to determine the efficacy and patient acceptability of the Paxman Scalp Cooler to prevent hair loss in patients receiving chemotherapy. European Journal of Oncology Nursing, 8(2), 121–130. https://doi.org/10.1016/j.ejon.2003.10.006
Nangia, J., Wang, T., Osborne, C., Niravath, P., Otte, K., Papish, S., . . . Rimawi, M. (2017). Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: The SCALP randomized clinical trial. JAMA, 317(6), 596–605. https://doi.org/10.1001/jama.2016.20939
Oshima, H., Rochat, A., Kedzia, C., Kobayashi, K., & Barrandon, Y. (2001). Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell, 104(2), 233–245. https://doi.org/10.1016/s0092-8674(01)00208-2
Pae, C.U. (2015). Why systematic review rather than narrative review? Psychiatry Investigation, 12(3), 417–419.
Page, M.J., McKenzie, J.E., Bossuyt, P.M., Boutron, I., Hoffmann, T.C., Mulrow, C.D., . . . Moher, D. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Journal of Clinical Epidemiology, 134, 178–189. https://doi.org/10.1016/j.jclinepi.2021.03.001
Panieri, E., & Santoro, M.M. (2016). ROS homeostasis and metabolism: A dangerous liason in cancer cells. Cell Death and Disease, 7(6), e2253. https://doi.org/10.1038/cddis.2016.105
Paus, R., Haslam, I.S., Sharov, A.A., & Botchkarev, V.A. (2013). Pathobiology of chemotherapy-induced hair loss. Lancet Oncology, 14(2), e50–e59. https://doi.org/10.1016/S1470-2045(12)70553-3
Pliskow, B., Mitra, K., & Kaya, M. (2016). Simulation of scalp cooling by external devices for prevention of chemotherapy-induced alopecia. Journal of Thermal Biology, 56, 31–38. https://doi.org/10.1016/j.jtherbio.2015.12.001
Roe, H. (2014). Scalp cooling: Management option for chemotherapy-induced alopecia. British Journal of Nursing, 23(16, Suppl.), S4–S12. https://doi.org/10.12968/bjon.2014.23.Sup16.S4
Ross, M., & Fischer-Cartlidge, E. (2017). Scalp cooling: A literature review of efficacy, safety, and tolerability for chemotherapy-induced alopecia. Clinical Journal of Oncology Nursing, 21(2), 226–233. https://doi.org/10.1188/17.CJON.226-233
Rubio-Gonzalez, B., Juhász, M., Fortman, J., & Mesinkovska, N.A. (2018). Pathogenesis and treatment options for chemotherapy-induced alopecia: A systematic review. International Journal of Dermatology, 57(12), 1417–1424. https://doi.org/10.1111/ijd.13906
Rugo, H.S., & Voigt, J. (2018). Scalp hypothermia for preventing alopecia during chemotherapy. A systematic review and meta-analysis of randomized controlled trials. Clinical Breast Cancer, 18(1), 19–28. https://doi.org/10.1016/j.clbc.2017.07.012
Rugo, H.S., Melin, S.A., & Voigt, J. (2017). Scalp cooling with adjuvant/neoadjuvant chemotherapy for breast cancer and the risk of scalp metastases: Systematic review and meta-analysis. Breast Cancer Research and Treatment, 163(2), 199–205. https://doi.org/10.1007/s10549-017-4185-9
Shah, V.V., Wikramanayake, T.C., DelCanto, G.M., van den Hurk, C., Wu, S., Lacouture, M.E., & Jimenez, J.J. (2018). Scalp hypothermia as a preventative measure for chemotherapy-induced alopecia: A review of controlled clinical trials. Journal of the European Academy of Dermatology and Venereology, 32(5), 720–734. https://doi.org/10.1111/jdv.14612
Shea, B.J., Grimshaw, J.M., Wells, G.A., Boers, M., Andersson, N., Hamel, C., . . . Bouter, L.M. (2007). Development of AMSTAR: A measurement tool to assess the methodological quality of systematic reviews. BMC Medical Research Methodology, 7, 10. https://doi.org/10.1186/1471-2288-7-10
Shea, B.J., Reeves, B.C., Wells, G., Thuku, M., Hamel, C., Moran, J., . . . Henry, D.A. (2017). AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ, 358, j4008. https://doi.org/10.1136/bmj.j4008
Shin, H., Jo, S.J., Kim, D.H., Kwon, O., & Myung, S.K. (2015). Efficacy of interventions for prevention of chemotherapy-induced alopecia: A systematic review and meta-analysis. International Journal of Cancer, 136(5), E442–E454. https://doi.org/10.1002/ijc.29115
Simon, H.U., Haj-Yehia, A., & Levi-Schaffer, F. (2000). Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis, 5(5), 415–418. https://doi.org/10.1023/a:1009616228304
Soref, C.M., & Fahl, W.E. (2015). A new strategy to prevent chemotherapy and radiotherapy-induced alopecia using topically applied vasoconstrictor. International Journal of Cancer, 136(1), 195–203. https://doi.org/10.1002/ijc.28961
Stewart, L., Moher, D., & Shekelle, P. (2012). Why prospective registration of systematic reviews makes sense. Systematic Reviews, 1, 7. https://doi.org/10.1186/2046-4053-1-7
Tosti, A., Piraccini, B.M., Vincenzi, C., & Misciali, C. (2005). Permanent alopecia after busulfan chemotherapy. British Journal of Dermatology, 152(5), 1056–1058. https://doi.org/10.1111/j.1365-2133.2005.06469.x
Trüeb, R.M. (2009). Chemotherapy-induced alopecia. Seminars in Cutaneous Medicine and Surgery, 28(1), 11–14. https://doi.org/10.1016/j.sder.2008.12.001
Trüeb, R.M. (2010). Chemotherapy-induced alopecia. Current Opinion in Supportive and Palliative Care, 4(4), 281–284. https://doi.org/10.1097/SPC.0b013e3283409280
van den Hurk, C.J., Peerbooms, M., van de Poll-Franse, L.V., Nortier, J.W., Coebergh, J.W.W., & Breed, W.P. (2012). Scalp cooling for hair preservation and associated characteristics in 1411 chemotherapy patients: Results of the Dutch Scalp Cooling Registry. Acta Oncologica, 51(4), 497–504. https://doi.org/10.3109/0284186X.2012.658966
van den Hurk, C.J.G., Mols, F., Vingerhoets, A.J.J.M., & Breed, W.P.M. (2010). Impact of alopecia and scalp cooling on the well-being of breast cancer patients. Psycho-Oncology, 19(7), 701–709. https://doi.org/10.1002/pon.1615
van den Hurk, C.J.G., van den Akker-van Marle, M.E., Breed, W.P.M., van de Poll-Franse, L.V., Nortier, J.W.R., & Coebergh, J.W.W. (2013). Impact of scalp cooling on chemotherapy-induced alopecia, wig use and hair growth of patients with cancer. European Journal of Oncology Nursing, 17(5), 536–540. https://doi.org/10.1016/j.ejon.2013.02.004
Wang, S., Yang, T., Shen, A., Qiang, W., Zhao, Z., & Zhang, F. (2021). The scalp cooling therapy for hair loss in breast cancer patients undergoing chemotherapy: A systematic review and meta-analysis. Supportive Care in Cancer, 29(11), 6943–6956. https://doi.org/10.1007/s00520-021-06188-8
Zhou, T., Han, S., Zhu, Z., Hu, Y., & Xing, W. (2020). Interventions for preventing chemotherapy-induced alopecia: A systematic review and network meta-analysis of randomized controlled trials. Cancer Nursing, 44(6), E567–E577. https://doi.org/10.1097/NCC.0000000000000899




