Exploring Daily Salivary Cortisol Patterns as Biomarkers of Chronic Chemotherapy-Induced Peripheral Neuropathy Pain
Objectives: Little is known about the biologic mechanisms of chronic chemotherapy-induced peripheral neuropathy (CIPN) pain. The purpose of this secondary analysis was to explore salivary cortisol patterns among cancer survivors with chronic CIPN pain to provide preliminary data regarding the role of hypothalamic-pituitary-adrenal axis dysregulation in the pathophysiology of this condition.
Sample & Setting: 13 cancer survivors with chronic CIPN pain recruited from the breast, gastrointestinal, and gynecologic cancer centers at Dana-Farber Cancer Institute in Boston, Massachusetts.
Methods & Variables: Salivary cortisol was collected on awakening, 30 minutes after awakening, and before going to bed on two consecutive days. Cortisol awakening response and diurnal cortisol slope were calculated by averaging results across two days.
Results: Cortisol was available from 13 participants. The median cortisol awakening response was –0.03 mcg/dl, and the average diurnal cortisol slope was –0.24 mcg/dl.
Implications for Nursing: Mechanism-based treatments are needed for cancer survivors with chronic CIPN pain. Nurse scientists may use study results to explore stress-related mechanisms of chronic CIPN pain.
Jump to a section
Chemotherapy-induced peripheral neuro-pathy (CIPN) is a disabling side effect of neurotoxic chemotherapy that causes neuropathic symptoms (e.g., numbness, tingling, shooting/burning pain) in the upper and lower extremities (Winters-Stone et al., 2017). Duloxetine (60 mg per day) is the only recommended drug for CIPN pain, and the strength of recommendation for its use is moderate (Loprinzi et al., 2020). Little is known about the mechanisms of underlying chronic CIPN pain, making it difficult to develop mechanism-based treatments for this condition.
Although neurotoxic chemotherapy agents have varying antineoplastic mechanisms of action, all are believed to directly or indirectly invoke a dying-back axonopathy that is responsible for the development of CIPN (Fukuda et al., 2017). Chronic CIPN results from permanent changes in the structure and functioning of the central nervous system and is therefore defined as neuropathic pain (Baron et al., 2010). One potential aspect of chronic CIPN development is dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, which is a primary regulator of the stress response (Eller-Smith et al., 2018). In healthy adults, HPA axis activation results in the release of cortisol to decrease peripheral inflammation (Eller-Smith et al., 2018). HPA axis activation ceases when cortisol binds to receptors in the hippocampus (Eller-Smith et al., 2018). However, in response to chronic stress, such as peripheral nerve injury, processes associated with HPA axis dysregulation (e.g., amygdala activation, increased corticotropin releasing factor) are initiated and result in cortisol dysfunction (Eller-Smith et al., 2018). In the absence of functioning cortisol, peripheral nerve injury results in unchecked peripheral inflammation and increased sensitization of peripheral nociceptors (Eller-Smith et al., 2018). Subsequently, peripheral sensitization results in continual nociceptive input from the chemotherapy-damaged nerve to the spinal cord dorsal horn, leading to neuronal hyperexcitability and decreased Aβ/Aδ fiber activation thresholds in a process called central sensitization (Latremoliere & Woolf, 2009).
One way to clinically assess HPA axis dysregulation in cancer survivors is to measure salivary cortisol (Hulett et al., 2019). Cortisol levels in healthy adults increase by approximately 50% from the time of awakening to 30–45 minutes after awakening (Adam & Kumari, 2009) and decrease gradually to their lowest point at bedtime (Adam et al., 2017). Evidence from a systematic review and meta-analysis of 80 studies (N = 36,823 participants) suggested that a flatter diurnal cortisol slope (smaller decreases between cortisol at awakening and bedtime) is significantly associated with worse physical and mental health outcomes (d = 0.147, p < 0.001 across all outcomes) (Adam et al., 2017). Data evaluating HPA axis dysregulation, as quantified by salivary cortisol levels, in cancer survivors with chronic CIPN are limited. The purpose of this secondary analysis was to quantify salivary cortisol levels among cancer survivors with chronic CIPN pain.
Methods
Design, Setting, and Sample
The data for this secondary analysis originated from a pilot, randomized controlled trial primarily designed to determine the feasibility of implementing a yoga intervention for cancer survivors with chronic CIPN pain (Knoerl et al., 2021). The pilot, randomized controlled yoga trial was guided by the Theory of Unpleasant Symptoms (Lenz et al., 1997), and salivary cortisol to measure HPA axis dysregulation was included as a potential physiologic influencing factor of chronic CIPN pain. Participants were recruited from the outpatient disease centers at Dana-Farber Cancer Institute in Boston, Massachusetts, using convenience sampling procedures. Patients were identified via weekly screening of electronic health record and clinician referral. The sample consisted of 44 English-speaking cancer survivors with breast, gastrointestinal, or gynecologic cancer who were experiencing chronic CIPN pain. Chronic CIPN pain was defined as experiencing CIPN for three months or more following neurotoxic chemotherapy completion and reporting a worst CIPN pain intensity score of 4 or higher during the past week on a numeric scale ranging from 0 (no pain) to 10 (worst pain). Participants were eligible for the secondary analysis if they completed at least one day of the baseline salivary cortisol measures.
Data Collection
Participants provided six salivary cortisol samples within 10 days following consent. Cortisol was quantitatively profiled from saliva using a competitive immunoassay kit. On two consecutive days, participants provided three salivary cortisol samples (on awakening, 30 minutes after awakening, and before going to bed) (Adam & Kumari, 2009) using an oral swab. Participants froze all samples at home prior to shipping the samples to the Brigham Research Assay Core. Cortisol analyses were conducted by the Brigham Research Assay Core in Boston, Massachusetts. The intra-assay variation of testing analyses at the laboratory is 3%–7%, and the inter-assay variation is 3%–11%. The lower limit of sensitivity is 0.007 mcg/dl. Cortisol collection was halted after the first 21 participants had been enrolled in the yoga trial because of COVID-19 transmission concerns. Baseline samples of salivary cortisol from 13 participants were analyzed in the current study.
Statistical Considerations
The secondary analysis included participants who completed at least one day of the baseline salivary cortisol measures. The cortisol awakening response was defined as the change in cortisol from awakening to 30–45 minutes after awakening, and diurnal cortisol slope was defined as the difference in cortisol between awakening and before going to bed (Adam & Kumari, 2009). These were both calculated by averaging the results across the two days and summarized using descriptive statistics.
Results
Participant Characteristics
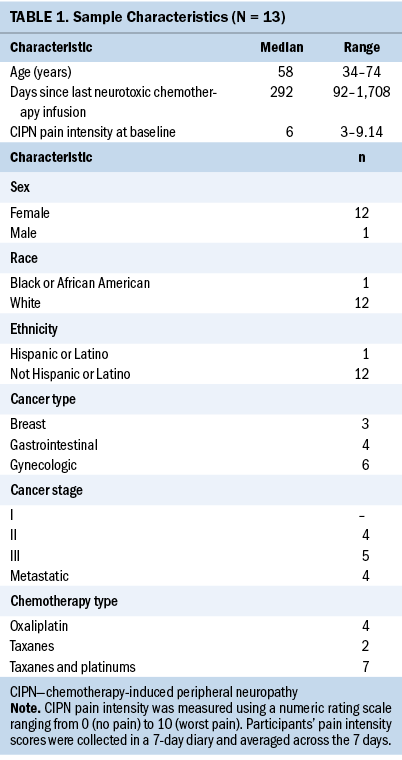
Of the 44 randomized and eligible participants in the primary trial (Knoerl et al., 2021), salivary cortisol data were available from 13 participants at baseline. Table 1 describes the demographic characteristics of the participants included in this secondary analysis.
Cortisol Awakening Response and Diurnal Cortisol Slope
Table 2 summarizes participants’ salivary cortisol values on awakening, 30–45 minutes after awakening, and before bedtime across two consecutive days. The median cortisol awakening response at baseline was –0.03 mcg/dl (range = –0.18 to 0.71), and the median diurnal cortisol slope was –0.24 mcg/dl (range = –0.42 to –0.05). 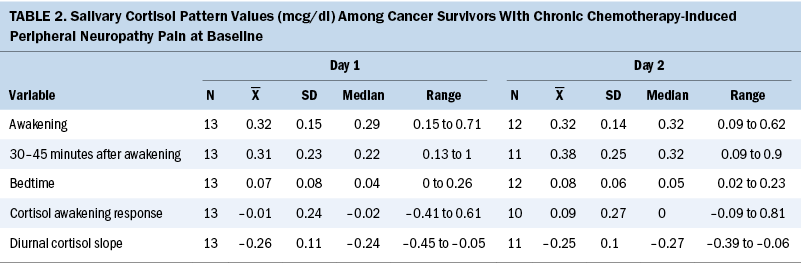
Discussion
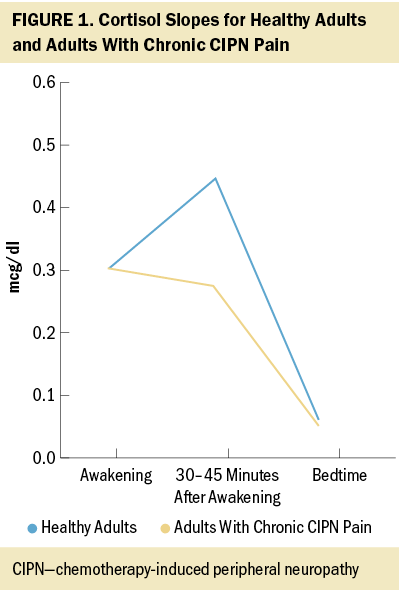
Baseline salivary cortisol study data revealed that cancer survivors with chronic CIPN pain exhibit an attenuated cortisol awakening response (about a 10% decrease) and flatter diurnal cortisol slope relative to healthy adults, potentially suggestive of HPA axis dysregulation (Adam & Kumari, 2009; Hulett et al., 2019) (see Figure 1).
Regarding the parent study and future directions for research, although the team planned to explore changes in salivary cortisol as a potential biomarker of HPA axis dysregulation following yoga in the pilot randomized controlled trial, the COVID-19 pandemic limited cortisol data collection, and few postintervention salivary cortisol samples were received (n = 5). However, preclinical research has demonstrated that exercise interventions may restore HPA axis dysregulation by increasing brain-derived neurotrophic factor (Maniam & Morris, 2010) or neurogenesis (Stranahan et al., 2007) in the hippocampus, thereby ceasing dysregulated HPA axis activity and subsequently reducing peripheral sensitization (Eller-Smith et al., 2018). Clinically, studies demonstrate that yoga significantly alters cortisol levels in individuals with chronic pain (Curtis et al., 2011).
Limitations
The analyses are exploratory in nature because of the high amount of missing data associated with salivary cortisol collection at baseline because of COVID-19 transmission concerns. In addition, other factors may have influenced participants’ salivary cortisol levels beyond CIPN alone (e.g., steroid use). For example, cross-sectional data demonstrate that a higher symptom burden (e.g., fatigue, hair loss, problems with sexual interest or activity) is associated with increased perceived stress among cancer survivors (N = 623) (Mazor et al., 2019).
Implications for Nursing
Nurse scientists may use these results to inform future research exploring stress-related biomarkers of chronic CIPN pain. Results of future research to investigate whether salivary cortisol awakening response and diurnal cortisol slope are predictive biomarkers of the efficacy of supportive care interventions for chronic CIPN pain will provide new evidence surrounding a clinically useful biomarker and intervention target for chronic CIPN pain. 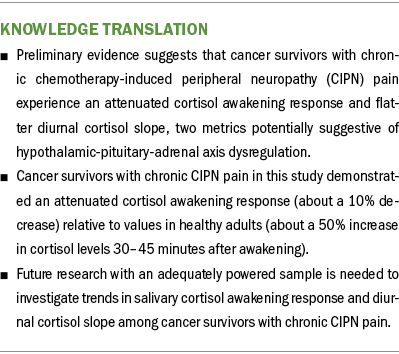
Conclusion
The preliminary results of this study suggest that cancer survivors with chronic CIPN pain demonstrate an attenuated cortisol awakening response and flatter diurnal cortisol slope at baseline, suggesting that HPA axis dysregulation may be a contributing pathophysiologic mechanism to chronic CIPN pain. Additional research in a larger sample of cancer survivors with chronic CIPN pain is needed to support the salivary cortisol patterns identified in this research. If further research supports the preliminary results, investigators may be able to measure the effectiveness of interventions targeting HPA axis dysregulation for the treatment of chronic CIPN pain.
The authors gratefully acknowledge Erica Fox, RN, Raymond Lamothe, MPH, Barbara Halpenny, MA, and Anna Tanasijevic, MPH, for their assistance with project administration.
About the Author(s)
Robert Knoerl, PhD, RN, is an assistant research scientist and assistant professor in the School of Nursing at the University of Michigan in Ann Arbor, and when the research described in this article was conducted, he was an instructor in medicine at the Phyllis F. Cantor Center for Research in Nursing and Patient Care Services at the Dana-Farber Cancer Institute; Anita Giobbie-Hurder, MS, is a biostatistician in the Division of Biostatistics in the Department of Data Science, and Juliana Berfield, PhD, is a yoga and fitness movement instructor in the Leonard P. Zakim Center for Integrative Therapies and Healthy Living, both at Dana-Farber Cancer Institute; Donna Berry, PhD, RN, AOCN®, FAAN, is a professor in Biobehavioral Nursing and Health Informatics at the University of Washington in Seattle; and Jeffrey A. Meyerhardt, MD, MPH, is a professor of medicine, and Alexi A. Wright, MD, MPH, and Jennifer A. Ligibel, MD, are associate professors at Harvard Medical School, all in the Department of Medical Oncology at Dana-Farber Cancer Institute. This research was funded by an Oncology Nursing Foundation Research Grant awarded to Knoerl. Knoerl has consulted for Strategy Inc., SPARK Healthcare, and System Analytic, and he serves on the scientific advisory board of Wellium. Meyerhardt has received institutional research funding from Boston Biomedical, served as an advisor/consultant to COTA Healthcare, and served on a grant review panel for the National Comprehensive Cancer Network funded by Taiho Pharmaceutical. Wright has served on the GlaxoSmithKline advisory board. All authors contributed to the conceptualization and design of the study and manuscript preparation. Knoerl completed the data collection. Giobbie-Hurder provided statistical support. Knoerl, Giobbie-Hurder, Berry, Wright, and Ligibel provided the analysis. Knoerl can be reached at rjknoerl@med.umich.edu, with copy to ONFEditor@ons.org. (Submitted July 2021. Accepted November 24, 2021.)
References
Adam, E.K., & Kumari, M. (2009). Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology, 34(10), 1423–1436. https://doi.org/10.1016/j.psyneuen.2009.06.011
Adam, E.K., Quinn, M.E., Tavernier, R., McQuillan, M.T., Dahlke, K.A., & Gilbert, K.E. (2017). Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology, 83, 25–41. https://doi.org/10.1016/j.psyneuen.2017.05.018
Baron, R., Binder, A., & Wasner, G. (2010). Neuropathic pain: Diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurology, 9(8), 807–819. https://doi.org/10.1016/S1474-4422(10)70143-5
Curtis, K., Osadchuk, A., & Katz, J. (2011). An eight-week yoga intervention is associated with improvements in pain, psychological functioning and mindfulness, and changes in cortisol levels in women with fibromyalgia. Journal of Pain Research, 4, 189–201. https://doi.org/10.2147/JPR.S22761
Eller-Smith, O.C., Nicol, A.L., & Christianson, J.A. (2018). Potential mechanisms underlying centralized pain and emerging therapeutic interventions. Frontiers in Cellular Neuroscience, 12(35), 1–18. https://doi.org/10.3389/fncel.2018.00035
Fukuda, Y., Li, Y., & Segal, R.A. (2017). A mechanistic understanding of axon degeneration in chemotherapy-induced peripheral neuropathy. Frontiers in Neuroscience, 11(481), 1–12. https://doi.org/10.3389/fnins.2017.00481
Hulett, J.M., Fessele, K.L., Clayton, M.F., & Eaton, L.H. (2019). Rigor and reproducibility: A systematic review of salivary cortisol sampling and reporting parameters used in cancer survivorship research. Biological Research for Nursing, 21(3), 318–334. https://doi.org/10.1177/1099800419835321
Knoerl, R., Giobbie-Hurder, A., Berfield, J., Berry, D., Meyerhardt, J.A., Wright, A.A., & Ligibel, J.A. (2021). Yoga for chronic chemotherapy-induced peripheral neuropathy pain: A pilot, randomized controlled trial. Journal of Cancer Survivorship, 1–10. https://doi.org/10.1007/S11764-021-01081-Z
Latremoliere, A., & Woolf, C.J. (2009). Central sensitization: A generator of pain hypersensitivity by central neural plasticity. Journal of Pain, 10(9), 895–926. https://doi.org/10.1016/j.jpain.2009.06.012
Lenz, E.R., Pugh, L.C., Milligan, R.A., Gift, A., & Suppe, F. (1997). The middle-range theory of unpleasant symptoms: An update. Advances in Nursing Science, 19(3), 14–27. https://doi.org/10.1097%2F00012272-199703000-00003
Loprinzi, C.L., Lacchetti, C., Bleeker, J., Cavaletti, G., Chauhan, C., Hertz, D.L., . . . Hershman, D.L. (2020). Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. Journal of Clinical Oncology, 38(28), 3325–3348. https://doi.org/10.1200/JCO.20.01399
Maniam, J., & Morris, M.J. (2010). Voluntary exercise and palatable high-fat diet both improve behavioural profile and stress responses in male rats exposed to early life stress: Role of hippocampus. Psychoneuroendocrinology, 35(10), 1553–1564. https://doi.org/10.1016/j.psyneuen.2010.05.012
Mazor, M., Paul, S.M., Chesney, M.A., Chen, L.-M., Smoot, B., Topp, K., . . . Miaskowski, C. (2019). Perceived stress is associated with a higher symptom burden in cancer survivors. Cancer, 125(24), 4509–4515. https://doi.org/10.1002/CNCR.32477
Stranahan, A.M., Khalil, D., & Gould, E. (2007). Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus, 17(11), 1017–1022. https://doi.org/10.1002/hipo.20348
Winters-Stone, K.M., Horak, F., Jacobs, P.G., Trubowitz, P., Dieckmann, N.F., Stoyles, S., & Faithfull, S. (2017). Falls, functioning, and disability among women with persistent symptoms of chemotherapy-induced peripheral neuropathy. Journal of Clinical Oncology, 35(23), 2604–2612. https://doi.org/10.1200/JCO.2016.71.3552

