An Integrative Review of Self-Management Interventions for Treatment Sequelae in Adult Survivors
Problem Identification: Self-management interventions support cancer survivors in addressing the consequences of treatment. With post-treatment survivors living longer, it is critical to know how research responds to their changing needs.
Literature Search: A comprehensive search of the CINAHL®, PsycINFO®, and PubMed® databases was performed. Articles were included if the self-management intervention was conducted on cancer-free adult survivors after completing primary treatment.
Data Evaluation: Each study was evaluated using the Critical Appraisal Skills Programme checklist.
Synthesis: 38 articles were included. The majority of the interventions were designed for short-term survivors, with limited interventions found to support the self-management of long-term cancer survivors. When implementing self-management support, there is a need to use theoretical frameworks that can respond to the changing needs of cancer survivors over time.
Implications for Practice: Future research should provide support for long-term survivors. Oncology nurses can use the results of this review to identify gaps in the self-management education provided to cancer survivors.
Jump to a section
Worldwide, the incidence of cancer is increasing (Bray et al., 2018). Although cancer mortality is increasing in many parts of the world, advancements in the prevention and treatment of cancer have also led to a dramatic increase in the number of people living with a history of cancer (de Moor et al., 2013). In the United States, an estimated 22.1 million people will be cancer survivors by 2030 (Miller et al., 2019). Cancer treatments improve survival, but they also can cause multiple side effects and consequences, including physical and psychological sequelae, many of which may not appear until years after treatment (Stein et al., 2008). Physical and psychological late and long-term sequelae—including chronic fatigue (Jones et al., 2016), cognitive dysfunction, anxiety, and depression (Gegechkori et al., 2017)—may exist for years after the completion of treatment.
For optimal management of late and long-term effects, cancer survivors need to integrate self-management behaviors into their lives (Foster & Fenlon, 2011). Post-treatment self-management can help survivors to address the long-term sequelae of treatment, as well as help them to adjust to life with cancer (McCorkle et al., 2011). Self-management refers to patients’ daily management of their chronic condition (Anekwe & Rahkovsky, 2018). Recommended self-management activities for individuals depend on the trajectory of their disease (Ryan & Sawin, 2009), but they generally involve three forms of activity: medical, emotional, and role management (Lorig & Holman, 2003). Medical management involves managing the medical components of a chronic disease, such as taking medications and adhering to the medication. Emotional management is related to psychosocial health and can include managing symptoms and emotions, such as depression, anxiety, anger, and uncertainty. Role management includes life roles, such as the ways in which individuals participate in a physical activity and their ability to fulfill roles within their relationships (e.g., parenting). Individuals involved in self-management require certain skills to successfully perform the self-management work. The skills include (a) decision making, (b) problem-solving, (c) using resources, (d) building partnerships between the patient and healthcare providers, (e) taking actions, and (f) self-tailoring (Lorig & Holman, 2003). Often, skills cut across many different types of self-management work. Rather than focus on skills and skills-building, self-management interventions usually target only one type of self-management work as their main focus of intervention.
Post-treatment cancer survivors need follow-up visits so that they can be monitored for treatment side effects and cancer recurrence. The American Cancer Society and the American Society of Clinical Oncology guidelines recommend that clinicians help cancer survivors in managing their health after completing treatment. The recommendations include screening for recurrence and second primary cancer, as well as managing the psychological and physical long-term consequences of treatment (Cohen et al., 2016; El-Shami et al., 2015; Runowicz et al., 2016). Cancer survivors need to receive support in the development and maintenance of self-management skills, as well as engagement in self-management activities (Foster et al., 2016). Such support can be delivered by healthcare professionals and ideally consists of both medical and psychosocial support (Dwarswaard et al., 2016; Furler et al., 2008). When delivered properly, this support involves more than providing information; it requires concrete strategizing to help survivors integrate self-management into their daily lives (e.g., changing health behaviors). Self-management interventions can target both patient engagement and the support required to facilitate it. Interventions can comprise multiple components (Richardson et al., 2014), attending to medical, emotional, and role management needs (Bal et al., 2016) to equip survivors with the skills necessary to actively engage in self-management (Jonkman et al., 2016).
In this integrative review, the current authors evaluate how interventions support using self-management for post-treatment cancer survivors, with the goal being that future interventions can better promote these self-management behaviors. Previous reviews of self-management interventions for cancer survivors have broadly defined “survivorship,” including studies from all stages of the cancer continuum (i.e., diagnosis, treatment, and survival) (Hammer et al., 2015; Kim & Park, 2015). However, given patients’ changing health as they proceed through the cancer continuum (Dulaney et al., 2017), it is likely that individuals with newly diagnosed cancers have different self-management support needs than individuals who are many years out from their cancer-related treatment (Foster & Fenlon, 2011). As a result, individuals’ self-management approaches will need to change and adapt as well. During the diagnosis and treatment of cancer, individuals often focus on survival, whereas for post-treatment survivors, the focus ideally should shift to promoting general health, managing the effects of their cancer and treatment, and preventing subsequent cancers. In this review, the current authors focus specifically on the post-treatment survivorship period. Evaluating interventions that target the self-management of post-treatment cancer survivors is an essential step in developing and implementing successful care for individuals living longer with a history of cancer.
Previous reviews on self-management interventions in post-treatment cancer survivors examined studies of self-management randomized controlled trials (RCTs) targeting patients’ self-management (Boland et al., 2017; Kim et al., 2017) or reviewed the psychoeducational content of the interventions designed to support coping and adaptation for cancer survivors (Cuthbert et al., 2019). The reviews found that interventions did not have good sustainability (Boland et al., 2017), mainly targeted breast cancer survivors (Cuthbert et al., 2019; Kim et al., 2017), demonstrated only a moderate effect of the intervention on health-related quality of life (Boland et al., 2017; Kim et al., 2017), and showed that psychoeducational interventions mainly focus on teaching coping skills (Cuthbert et al., 2019). These reviews, although focused on synthesizing self-management interventions, did not examine how the current literature regarding self-management interventions has provided support for those who have completed treatment and are considered to be disease free.
The purpose of this integrative review is to provide a comprehensive examination of self-management interventions for post-treatment cancer survivors from studies with a range of designs. In doing so, the current authors illuminate how self-management is conceptualized and implemented among this growing population. The specific research questions for this review are as follows:
• How has the research on self-management considered the ways that cancer survivors and their needs change over time?
• What theoretical frameworks are used for designing self-management interventions, and how do these frameworks respond to the survivorship care and follow-up guidelines?
Methods
The current authors conducted a systematic search to review the literature. Articles were reviewed if the target population was adult survivors (aged 18 years or older) who had completed primary treatment (chemotherapy, radiation therapy, surgery) and were considered to be cancer free. Articles were included if (a) individuals were cancer free but received maintenance therapy, (b) the interventions targeted self-management in post-treatment cancer survivors, (c) the outcomes of the study were measured and reported, and (d) the articles were written in English. Studies that focused on interventions among survivors of childhood cancers and survivors who were in active treatment were not included.
Literature Search
Search strategies were developed with the assistance of one author (J.D.) who is a health sciences librarian with expertise in searching for integrative reviews. Searches were created by the librarian and primary author (S.S.) using an iterative process of gathering and evaluating terms. Initial searches were conducted in August and September 2019 and updated in January 2020 to identify new articles published during the screening process. Searches included the full publication range of databases. Comprehensive strategies, including both index and keyword methods, were devised for the following databases: CINAHL®, PsycINFO®, and PubMed®. To maximize sensitivity, no preestablished filters were used. However, customized filters designed to identify intervention studies were applied in all databases. The full PubMed search strategy was adapted for use with the other electronic databases. Search strategies are available on request. In addition to the database searches described, in January 2020, citing articles and reference lists were screened from a set of identified relevant articles to ensure that all potential references had been identified.
Study Selection
After removing the duplicates, one author (S.S.) screened titles and abstracts for all the articles twice. Studies that did not explicitly define their populations as post-treatment and cancer-free (i.e., studies that indicated only no evidence of metastasis) were excluded, as were those that did not align with Lorig’s definition of self-management, a well-established definition that focuses on self-management activities and skills (Lorig & Holman, 2003). Studies on adaptation and coping were not included because these concepts are different from self-management (Audulv et al., 2016). Thirty-two articles were found to be eligible and were entered into a literature matrix. Data were extracted from the articles based on the inclusion criteria. In cases where articles were not clearly eligible or ineligible, they were reviewed by a second author (S.G.-W.). Agreement over inclusion was reached by consensus.
Critical Appraisal of Individual Studies
Critical Appraisal Skill Programme (CASP) checklists were used to assess the quality of RCT and non-RCT studies at the study level. Two CASP checklists for cohort and RCT studies were used. The cohort study checklist has 12 questions, and the RCT checklist has 11 questions. Each item on the checklist has three responses: yes, cannot tell, and no (CASP UK, n.d.). Studies scored between 45% and 89% on the checklist. Although some studies were evaluated as being of lower quality based on CASP criteria, the current authors did not exclude them because they still provided valuable data for future self-management intervention design.
Results
Study Characteristics
After duplicates were removed, a total of 2,976 articles were screened. Of these, 2,901 were excluded because they were not self-management interventions within the defined parameters and/or they did not target post-treatment cancer survivors. The remaining 75 full-text articles were assessed for eligibility, and 38 articles representing as many studies were included in the analysis (see Figure 1). Publication dates for the included studies ranged from 1983 to 2020. The majority of the self-management interventions were RCTs (n = 28), whereas less than one-third (n = 10) were non-RCTs. The total sample size of individual studies ranged from 10 to 625. Time since survivorship ranged from one week to five or more years. Thirty interventions were designed for short-term survivors. The short-term survivorship (less than five years) period ranged from 1 to 56 weeks. Three interventions targeted the self-management of long-term survivors, with the time since survivorship being five or more years. Twelve of the studies did not specify the time since survivorship for their study population. 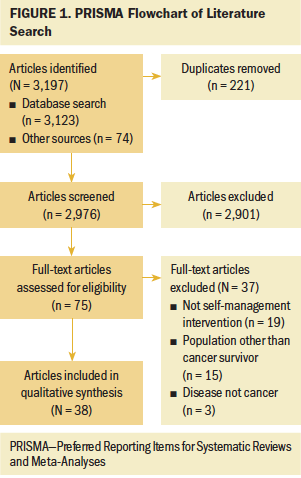
The mean age of participants in individual studies ranged from 37.6 years (SD = 8) to 71.1 years (SD = 3.6) (see Table 1). The two most common cancer types targeted by the interventions were breast and colorectal. Participants’ cancer stage ranged from stage 0 to IV. None of the studies specifically targeted rural cancer survivors, and gender was not diverse beyond male and female. 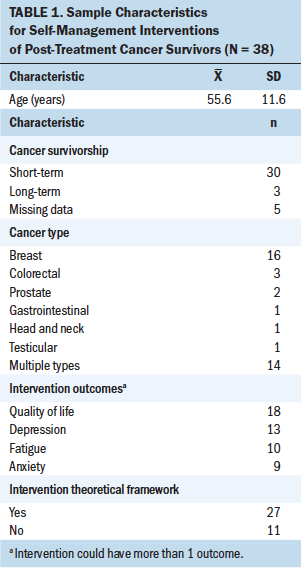
Intervention Characteristics
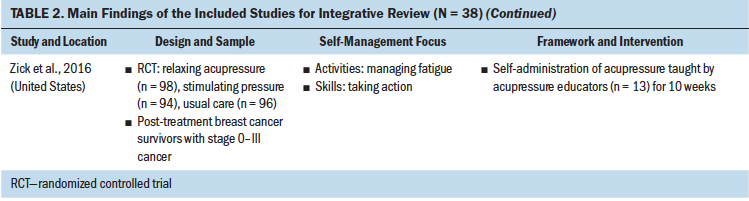
Study interventions varied in delivery method, duration, and type of interventionist delivering the self-management support. Delivery methods implemented by the studies to support self-management included one-on-one individual sessions, group sessions, technology based, combination of multiple methods, and in person or home based. A few studies did not specify the delivery method. The educational materials either were delivered through face-to-face lecture or were provided to the participants to self-study at home. Nurses, mental health professionals, social workers, and physiotherapists were among those delivering the interventions. The duration of the interventions ranged from one session to six months. Twenty-nine interventions targeted physical aspects of self-management, such as exercising, and nine targeted the psychosocial aspects of self-management, such as managing mood and changing cognition (see Table 2). 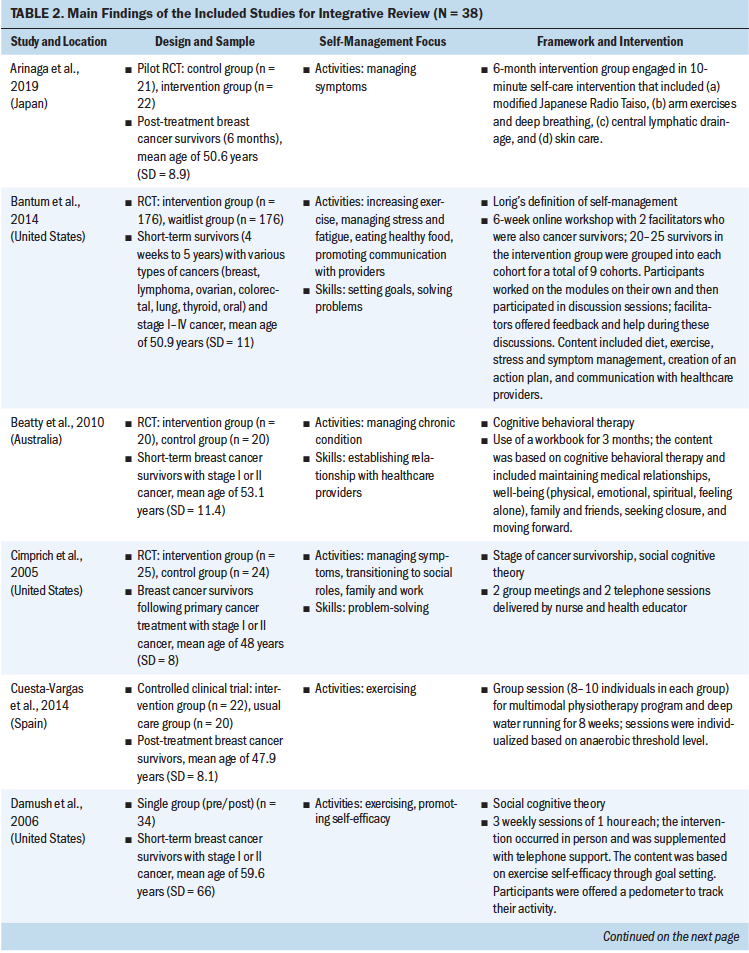
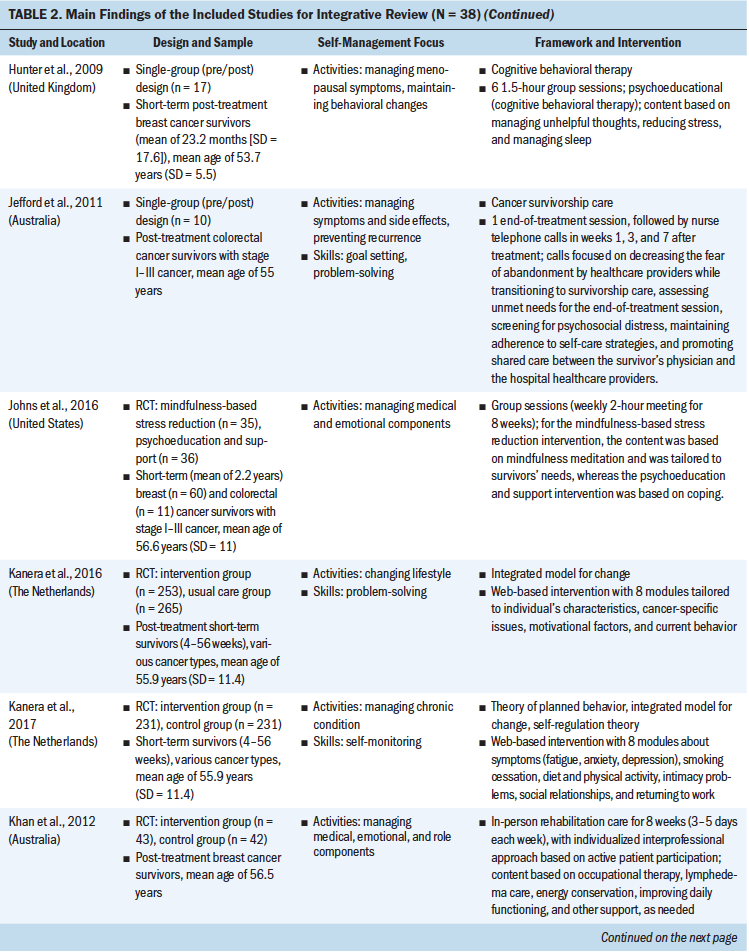
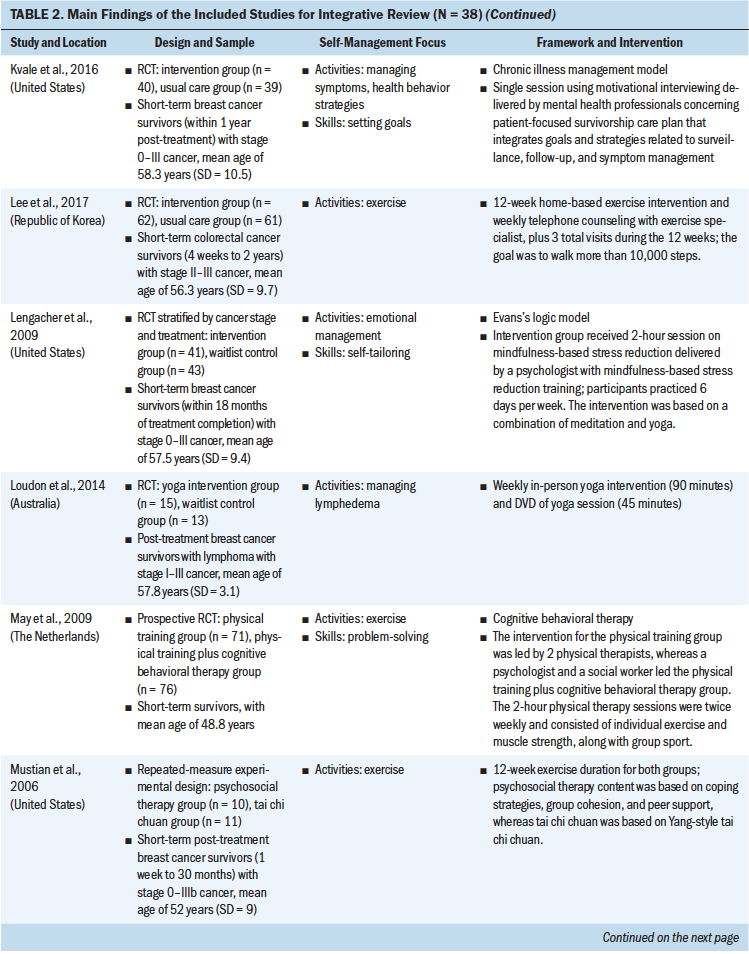
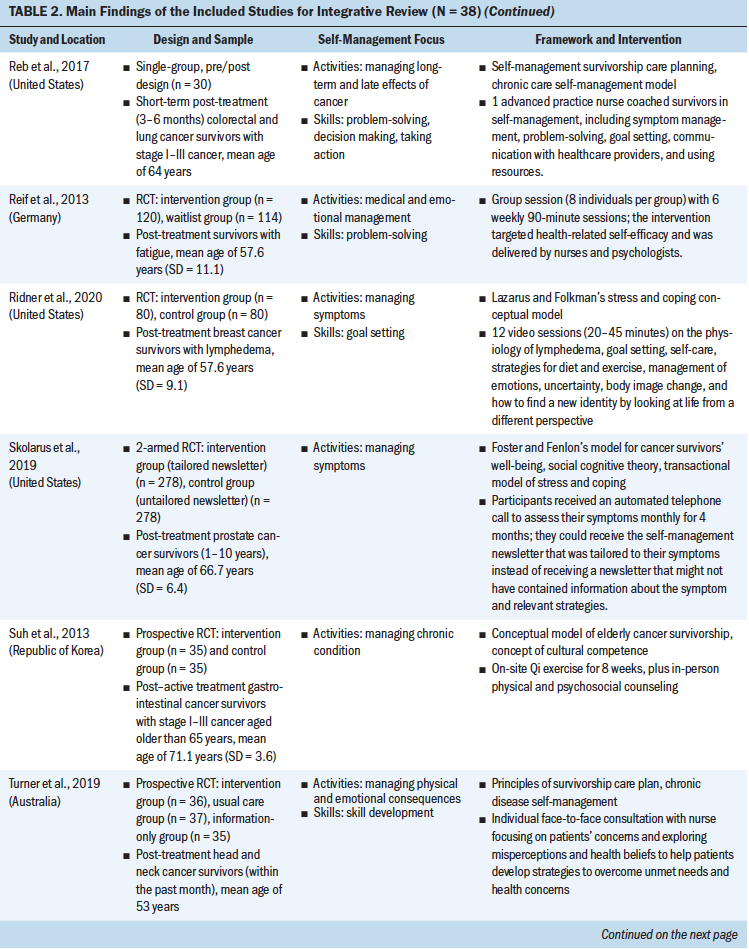
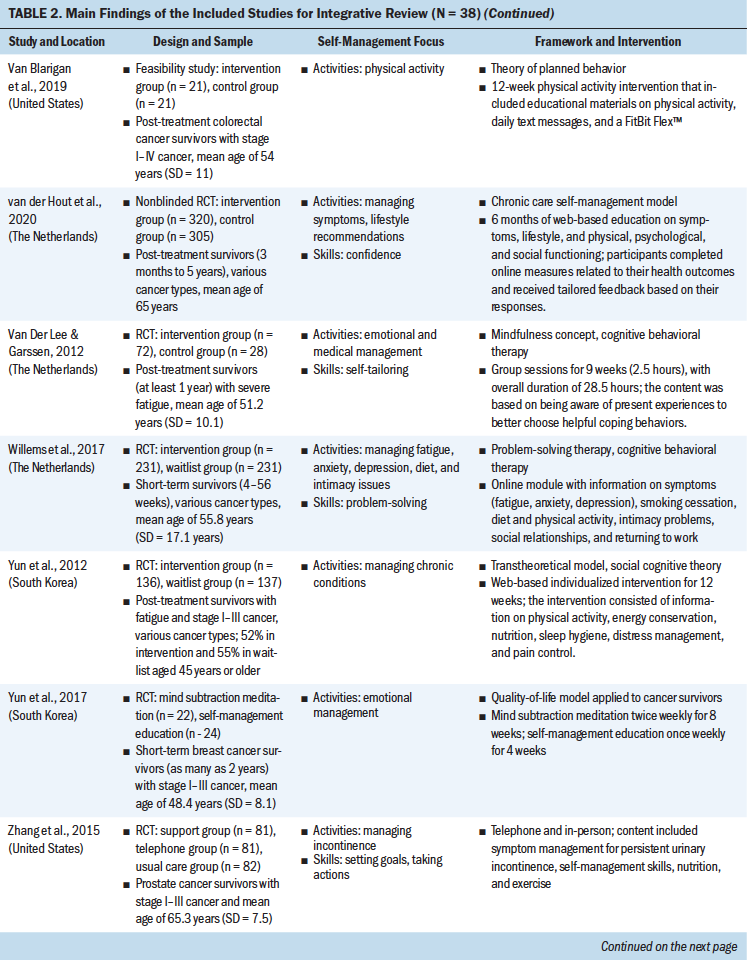
Theoretical Frameworks and Principles
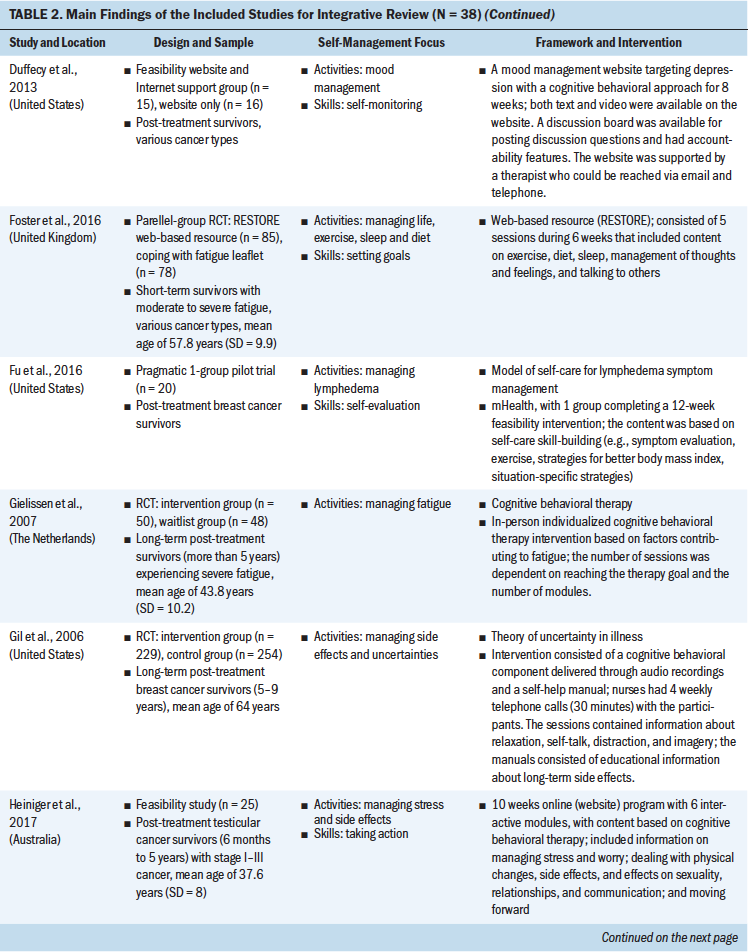
Review of the results showed that 27 interventions used theoretical or conceptual models to guide their study. Types of theories and frameworks to guide the intervention were related to self-management, cancer survivorship, and principles of cognitive behavioral therapy. The self-management and health behavior theories focused on supporting self-management activities and skills, such as managing uncertainty (Gil et al., 2006), managing lymphedema (Fu et al., 2016), changing health behavior (Kanera et al., 2016, 2017; Yun et al., 2012), improving overall self-management activities and skills (Bantum et al., 2014; Kvale et al., 2016), meditating (Lengacher et al., 2009), and managing stress and coping (Ridner et al., 2020). Survivorship models detailed post-treatment health needs, such as the role of time since survivorship (acute, extended, and permanent survival) (Cimprich et al., 2005), quality of life (Yun et al., 2017), the needs of older adult cancer survivors (Suh et al., 2013), overall cancer survivorship care (Jefford et al., 2011), and cancer survivors’ well-being (Foster et al., 2016; Skolarus et al., 2019). Cognitive behavioral theories focused on symptom management and exercise (Beatty et al., 2010; Damush et al., 2006; Gielissen et al., 2007; Heiniger et al., 2017; Hunter et al., 2009; May et al., 2009; van der Lee & Garssen, 2012; Willems et al., 2017).
Self-Management Focus
To review the self-management focus, the results were organized based on the type of self-management activity and skill, outlined by a well-established interprofessional definition of self-management (Lorig & Holman, 2003). The support provided for self-management activities represents delivering education regarding the medical, emotional, and role management aspects of cancer survivorship, and self-management skills reflect teaching the necessary skills for engaging in self-management activities. Although 23 interventions supported participants in learning both self-management activities and skills, 15 provided support in learning self-management activities only. Some self-management interventions broadly targeted self-management activities through providing education on overarching medical, emotional (Bantum et al., 2014; Beatty et al., 2010; Heiniger et al., 2017; Jefford et al., 2011; Johns et al., 2016; Kanera et al., 2017; Reif et al., 2013; Suh et al., 2013; van der Lee & Garssen, 2012; Willems et al., 2017; Yun et al., 2012), and role (Jefford et al., 2011) management, but the majority of the interventions specifically targeted medical or emotional management.
These self-management interventions mainly focused on medical management through symptom management (Cimprich et al., 2005; Hunter et al., 2009; Jefford et al., 2011; Kvale et al., 2016), including fatigue (Gielissen et al., 2007; Zick et al., 2016), mood (Duffecy et al., 2013), lymphedema (Fu et al., 2016; Loudon et al., 2014), and incontinence (Zhang et al., 2015), as well as through management of side effects (e.g., stiffness) (Gil et al., 2006) and late effects (e.g., change in bowel pattern) of cancer (Reb et al., 2017). As part of medical management, some interventions also focused on pursuing healthy behaviors (May et al., 2009), exercise (Cuesta-Vargas et al., 2014; Damush et al., 2006; Lee et al., 2017; May et al., 2009; Mustian et al., 2006), and changing lifestyle (Kanera et al., 2016). The type of exercises used in the interventions were Qi (Suh et al., 2013), home based (Lee et al., 2017), tai chi chuan (Mustian et al., 2006), and deep water running (Cuesta-Vargas et al., 2014). The emotional management interventions (Lengacher et al., 2009) were related to uncertainty management (Gil et al., 2006), mindfulness, coping behaviors (van der Lee & Garssen, 2012), and mind subtraction meditation (Yun et al., 2017).
Some of the self-management interventions incorporated skill-building in their programs. These self-management skills were related to problem-solving (Bantum et al., 2014; Cimprich et al., 2005; Jefford et al., 2011; Kanera et al., 2016; Reb et al., 2017; Reif et al., 2013; Willems et al., 2017), setting goals (Bantum et al., 2014; Foster et al., 2016; Kvale et al., 2016), taking actions (Bantum et al., 2014; Heiniger et al., 2017; Zhang et al., 2015; Zick et al., 2016), self-tailoring (Bantum et al., 2014; Fu et al., 2016; Lengacher et al., 2009; van der Lee & Garssen, 2012), establishing relationships with healthcare providers (Beatty et al., 2010), and self-monitoring (Duffecy et al., 2013; Kanera et al., 2017). The majority of the studies that integrated skills in their self-management interventions were guided by cognitive behavioral therapy (Beatty et al., 2010; Cimprich et al., 2005; Foster et al., 2016; Heiniger et al., 2017; May et al., 2009; van der Lee & Garssen, 2012; Willems et al., 2017).
Outcome Measures and Effect of Self-Management Intervention
Surveys were commonly used to collect data related to the effects of self-management interventions; the use of a physiological measure (e.g., biomarker) was infrequent. Only one study (Lee et al., 2017) used adiponectin and tumor necrosis factor alpha to measure the impact of exercise. Generic and cancer-specific quality-of-life surveys were used, with most studies (n = 18) targeting quality of life as their primary outcome. Depression was the second most frequent outcome measure (n = 13).
Overall, self-management interventions improved the targeted health outcomes (Cuesta-Vargas et al., 2014; Reb et al., 2017; Suh et al., 2013). Interventions were effective in lowering symptoms such as depression and/or anxiety (Duffecy et al., 2013; Khan et al., 2012; Lengacher et al., 2009; Reif et al., 2013; Yun et al., 2012, 2017), fatigue level (Reif et al., 2013; van der Lee & Garssen, 2012; Willems et al., 2017; Yun et al., 2012; Zick et al., 2016), tissue induration for lymphedema (Loudon et al., 2014), and symptom severity (Fu et al., 2016; Zhang et al., 2015). The self-management interventions also improved venting coping (Beatty et al., 2010), quality of life (Reif et al., 2013), insomnia (Bantum et al., 2014), exercise level (Bantum et al., 2014; Kanera et al., 2016, 2017; Lee et al., 2017), vitality (Johns et al., 2016), vegetable consumption (Kanera et al., 2016), and overall health and self-efficacy (Kvale et al., 2016), including fatigue self-efficacy (Foster et al., 2016).
Discussion
This integrative review offers important information regarding self-management interventions for post-treatment cancer survivors. Cancers targeted by these interventions are among the most common cancers diagnosed worldwide (Bray et al., 2018). Indeed, the majority of the self-management interventions were designed exclusively for breast cancer survivors. A small number of studies were designed for male-specific cancers; only three studies specifically targeted survivors of prostate cancer (Skolarus et al., 2019; Zhang et al., 2015) and testicular cancer (Heiniger et al., 2017). Although the survival rate for stage IV cancer is typically low, when individuals survive, they are left with severe consequences that need to be managed (Perwein et al., 2011). However, only one intervention (Van Blarigan et al., 2019) was found to target post-treatment survivors with stage IV diagnoses. Noticeably, none of the studies exclusively targeted survivors living in rural areas who may have reduced access to survivorship management care, which is more common in urban areas and/or areas with academic medical centers. In addition, only three studies focused on the survivorship experience of long-term, post-treatment survivors (Gielissen et al., 2007; Gil et al., 2006; Skolarus et al., 2019). It is difficult to synthesize how the specific self-management needs of long-term cancer survivors were met. This is an important gap that needs to be addressed in the future by designing more interventions for long-term survivors. Reflecting a trend in cancer survivorship research more broadly (National Academies of Sciences, Engineering, and Medicine, 2018), these gaps suggest that future research into self-management for cancer survivors needs to attend to the diversity of survivor populations and their potentially distinct needs.
Studies that did not have a clear definition of post-treatment cancer survivors were excluded from this review. In these excluded studies, authors did not explicitly report if survivors were cancer free or had completed treatment. However, some of the studies that were ultimately excluded from this review targeted populations of survivors that are often understudied. For instance, Van Kampen et al. (2000) conducted a study involving patients with prostate cancer with the aim of managing urinary incontinence, which is one of the most common symptoms patients experience after prostatectomy. In another study, Lee et al. (2010), developed a tai chi exercise intervention for patients with stage I or II gastric cancer.
Designing a theory-guided intervention is necessary to delivering tailored self-management support. Overall, the majority of the interventions were guided by a theory or conceptual model. All reviewed theories and frameworks facilitated providing self-management support. However, only three interventions integrated elements of self-management in the context of post-treatment survivorship. Foster and Fenlon’s model for cancer survivors’ well-being (Foster et al., 2016) considers self-management strategies as part of the survivor’s well-being (Foster & Fenlon, 2011). The model of self-care for lymphedema symptom management was exclusively developed for post-treatment cancer survivors (Fu et al., 2016) and depicts the components of lymphedema self-care. One study used a framework that integrated survivorship care guidelines and self-management (Turner et al., 2019).
Based on these findings, the current authors recognize the need for additional development of self-management theories that can attend to the changing needs of post-treatment cancer survivors over time. During survivorship, post-treatment cancer survivors have two significant transition periods: (a) active treatment to short-term survival (less than five years), and (b) short-term survival to long-term survivorship (five or more years). Long-term cancer survivors have unique needs because they are often deemed cancer free (Bell & Ristovski-Slijepcevic, 2013), typically five years after completing cancer treatment. However, there is no guarantee that they will be free from the consequences of cancer and its treatment. Limited access to regular clinic visits (Fang & Lee, 2016) presses them to be independently responsible for maintaining and promoting their health. Because self-management has been recognized as a method for helping individuals help themselves (Dineen-Griffin et al., 2019), acknowledging that self-management occurs in the context of post-treatment cancer survivorship is critical. The current authors propose that building on this work to target post-treatment survivors is a crucial next step. In future development of self-management theories, researchers should consider (a) detailing the required self-management activities and skills for post-treatment survivors, (b) designing conceptual models that can be applied across cancer types, and (c) including a survivorship care guideline as part of the theory while not limiting the applicability of the models to the institutions that provide survivorship care planning.
Regarding the focus of self-management interventions, results of the current study showed that most interventions emphasized providing education relevant to medical management, such as managing symptoms and exercising. A few interventions targeted emotional or role management. Problem-solving was the primary skill targeted by self-management interventions. Most studies focused on teaching one skill, and only one study targeted all elements of self-management skills (Bantum et al., 2014). Studies that were guided by cognitive behavioral therapy helped patients to use problem-solving in the context of managing their health, but other interventions did not discuss how participants used skills for performing self-management activities. Therefore, it is difficult to discuss whether providing education on skills or preparing individuals to perform certain self-management activities creates a change in individuals’ approach to self-management.
The majority of studies used quality of life as the primary outcome measure. Interventions that were designed for long-term survivors focused on symptom management, mainly managing uncertainty (Gil et al., 2006), fatigue (Gielissen et al., 2007), and overall symptoms (Skolarus et al., 2019), whereas interventions designed for short-term survivors covered multiple domains of self-management, such as exercising and providing support for developing self-management skills. In studies with self-management interventions, no differences were found regarding design, content, and theories and/or frameworks for short-term and long-term survivors. Given the range of designs included, the current authors did not evaluate studies’ risk of bias, which limits their ability to comment on the outcomes. However, it is worth noting that the reported results for self-management interventions showed an improvement in health outcomes for patients, such as increased quality of life and decreased symptom severity. Noticeably, none of the studies measured how the intervention influenced survivors’ self-management practices after the intervention was completed. Although targeting the health-related outcome is desired, self-management interventions should also provide an opportunity to help survivors in their independent self-management practices after the intervention is completed. Self-management interventions can also benefit from using innovative outcome measures, such as biomarker measures, which can demonstrate a more precise method for measuring the effects of the intervention on health outcomes. All studies except one used surveys for measuring the outcomes of the self-management interventions. Lee et al. (2017) instead used biomarkers, including adiponectin and tumor necrosis factor alpha, for measuring the outcomes of an exercise intervention.
Limitations
Despite using a well-established definition of self-management, the absence of a classification system for self-management interventions made it difficult to categorize the focus of self-management interventions. Self-management studies for people living with advanced cancer who will not enter survivorship, as defined for this review, were not included. Although the current authors believe that the self-management needs of patients who are receiving antineoplastic treatments are different than those of patients in post-treatment survivorship, it is possible that advances in the self-management literature for patients with advanced cancer may provide insights relevant to post-treatment survivors. Similarly, studies of self-management for cancer survivors aged younger than 18 years were not included in this review; these may contain findings relevant to adult populations.
Although allowing for the important examination of self-management components, the heterogeneity of the included studies and their design (RCT versus non-RCT) reduces the ability to provide informative dosage recommendations for practice. In addition, the majority of interventions were conducted on breast cancer survivors; therefore, the results may not be applicable to other cancer survivors because the symptoms and side effects of breast cancer differ from those of other cancers, limiting recommendations concerning self-management interventions.
Implications for Practice
With a growing population of cancer survivors, oncology nurses play an important role in promoting their health and well-being. Although acknowledging that nurses in practice cannot teach all self-management activities and skills, the results showed that self-management support is effective in improving health outcomes. In practice, oncology nurses can help survivors identify their health concerns and provide resources to survivors to support their self-management practices. Nurses can use the results of the current study to educate survivors that any form of self-management activity, even if focused only on managing uncertainties, can help them to have better health outcomes. Oncology nurses and other healthcare providers can use the results of this review to identify current gaps in the self-management education provided to cancer survivors in the clinical setting.
With numerous challenges faced by cancer survivors after completing treatment, it is important that researchers develop self-management support specific to their health needs. Several opportunities exist for researchers to design innovative studies using theories and frameworks that address the specific needs of cancer survivors. Oncology researchers should consider designing self-management interventions for long-term survivors because this critical topic has been largely overlooked in the survivorship literature. 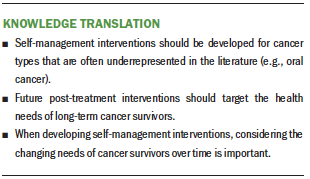
Conclusion
Several studies used theories and frameworks that were not specific to cancer. Although these broad theories and frameworks are useful, cancer-specific theories are needed to develop robust, targeted interventions. In designing future self-management interventions, it is recommended that the interventions consider including a diverse population (e.g., cancer type, gender) and identifying the self-management skills and activities that are specific to one’s phase of cancer survivorship (such as short-term survivors versus long-term survivors). This will allow researchers to better address the changing needs of cancer survivors over time.
About the Author(s)
Seyedehtanaz Saeidzadeh, PhD, RN, was, at the time of this writing, a PhD student, and Stephanie Gilbertson-White, PhD, APRN-BC, is an associate professor, both in the College of Nursing at the University of Iowa in Iowa City; Faezeh Babaieasl, PhD, MSN, RN, is an assistant professor in the School of Nursing at Babol University of Medical Sciences in Iran; Jennifer DeBerg, MLS, is a user services librarian in the Hardin Library for the Health Sciences at the University of Iowa Libraries in Iowa City; and Aaron T. Seaman, PhD, is an assistant professor of internal medicine at the University of Iowa in Iowa City. No financial relationships to disclose. Saeidzadeh, Gilbertson-White, DeBerg, and Seaman contributed to the conceptualization and design. Saeidzadeh, DeBerg, and Seaman completed the data collection. Seaman provided statistical support. Saeidzadeh, Gilbertson-White, and Seaman provided analysis. All authors contributed to the manuscript preparation. Saeidzadeh can be reached at saeidzadeh@wisc.edu, with copy to ONFEditor@ons.org. (Submitted February 2020. Accepted August 12, 2020.)




