ONS Guidelines™ for Cancer Treatment–Related Lymphedema
Purpose: Lymphedema is a chronic condition that may result from cancer-related surgery. The incidence of lymphedema varies greatly; however, patients remain at risk for life and may experience decreased quality of life and functional capacity. Providing recommendations for an evidence-based guideline for care of cancer treatment–related lymphedema will help to improve outcomes for patients with this chronic condition.
Methodologic Approach: A panel of healthcare professionals with patient representation convened to develop a national clinical practice guideline on prospective surveillance, risk reduction, and conservative treatment of lymphedema. Systematic reviews of the literature were conducted and the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) methodology approach was used to assess the evidence.
Findings: The panel made multiple recommendations for patients who are at risk for or experiencing lymphedema.
Implications for Nursing: Early diagnosis and treatment of lymphedema may mitigate symptoms. This evidence-based guideline supports patients, clinicians, and other healthcare professionals in clinical decision making.
Supplementary material can be found at https://onf.ons.org/supplementary-material-ons-guidelines-cancer-treatment-related-lymphedema.
Jump to a section
Secondary (acquired) lymphedema is a chronic condition most commonly resulting from cancer treatment (surgery, radiation therapy, and/or chemotherapy), affecting an estimated three to five million people in the United States and tens of millions worldwide (Lymphedema Education & Research Network, 2020; Sleigh & Manna, 2019). Lymphedema is characterized by an accumulation of protein-rich lymphatic fluid in the affected part of the body, potentially affecting function, psychological and physiologic factors, family roles and relationships, and occupational roles and productivity. Secondary lymphedema can be caused from surgical trauma to the lymphatic channels, radiation therapy, infection, scarring associated with wound healing, and compression of the lymphatics by tumors (Armer et al., 2004; Chang & Cormier, 2013; FÖldi & FÖldi, 2012; Lasinski et al., 2012; National Cancer Institute, 2019; Shah et al., 2012). Most commonly, secondary lymphedema is associated with cancer-related treatment for breast, gynecologic, prostate, lymphoma, melanoma, and head and neck cancers (National Cancer Institute, 2019). The most common cancer treatment–related lymphedema is associated with breast cancer (National Cancer Institute, 2019; Sleigh & Manna, 2019), in part related to the relatively high incidence and prevalence of breast cancer cases and the relatively high survival rate. The American Cancer Society (ACS) estimates 271,270 new breast cancer cases in 2019, of which an estimated 10% to 40% may develop breast cancer–related lymphedema (BCRL) (ACS, 2019; Armer & Stewart, 2010).
Lymphedema is a chronic condition, without a known cure, and survivors whose lymphatic systems are damaged by cancer treatment are considered at lifetime risk of developing lymphedema. Because of this lifetime risk, many surveillance and management approaches have been developed, most of which are nonpharmaceutical and use more than one modality for treatment.
A latent subclinical stage of lymphedema often precedes the chronic phase of lymphedema; surveillance for progression of the condition requires clinical observation of increased swelling, skin changes, and fibrosis. A study conducted by Boccardo et al. (2009) reported that about 75% of lymphedema cases occur in the first year after breast cancer surgery. There have been several studies conducted to report on various prospective surveillance interventions and frequencies (Shah et al., 2016). The interventions used in the prospective surveillance programs vary and include measurement, education, exercise, and symptom assessments (Box et al., 2002; Chance-Hetzler et al., 2015; Fu et al., 2014; Ostby et al., 2014; Ridner et al., 2019; Torres Lacomba et al., 2010).
Current standard of care for lymphedema treatment is complete decongestive therapy (CDT), including intensive lymphedema therapy (phase 1 of CDT) with a certified lymphedema therapist, followed by lifelong self-management (phase 2 of CDT) administered by the patient and/or a caregiver. The self-management of CDT phase 2 includes continued meticulous skin and nail care, range of motion exercises, manual lymphatic drainage (MLD), and application of compression garments and/or bandages. Follow-up includes periodic monitoring of self-care practices. Findings of soft tissue changes requires alterations in the self-care plan (Deng et al., 2019).
The aim of this guideline is to provide evidence-based lymphedema risk-reduction and symptom management recommendations for patients with cancer-related lymphedema. This guideline incorporates recently published research on interventions for prospective surveillance, risk reduction, and treatment for lymphedema following cancer-related surgery. The Oncology Nursing Society (ONS) Guidelines™ panel considers risk reduction to include the minimization or delay in developing secondary lymphedema while noting that patients have a lifetime risk of lymphedema following cancer surgery. The target audience includes oncology healthcare professionals, patients, and health policy decision makers. Policymakers interested in this guideline include individuals and organizations developing local, national, or international protocols with a goal of improving management for adults who are experiencing cancer treatment–related lymphedema. The guideline is based on systematic reviews and a network meta-analysis that explored this research topic.
Guideline Development Methods
ONS vetted and appointed individuals to the guideline panel. The membership of the panel included oncology nurses at all levels of practice, a lymphedema specialist, and a patient representative (see online Appendix for more information). The evidence synthesis for this guideline was based on two rigorously conducted systematic reviews (Ding et al., 2020; Lytvyn et al., 2020). The panel was coordinated by the senior manager of evidence-based practice at ONS (P.K.G.), with collaboration from a methodologist with expertise in evidence appraisal and guideline development (R.L.M.). The panel completed its work online using GRADEpro, a web-based tool, to aid with summarization and grading evidence, and met in person during a two-day final evidence review and recommendation consensus meeting.
The guideline panel assessed the certainty in the evidence and developed the recommendations according to the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) methodology approach (Guyatt, Oxman, Akl, et al., 2011). The guideline development process—including panel formation, management of conflicts of interest, internal and external review, and organizational approval—was guided by policies and procedures derived from the Guideline International Network–McMaster Guideline Development Checklist and the National Academies of Sciences, Engineering, and Medicine (NASEM) criteria for trustworthy guidelines (Institute of Medicine, 2011; Schünemann et al., 2014).
Financial and intellectual disclosures of interest of all participants were collected and managed according to ONS policies and the recommendations of the NASEM and the Guideline International Network (Institute of Medicine, 2011; Schünemann et al., 2015). At the time of appointment and again at the recommendations meeting, disclosures were recorded and the guideline panel had no relevant conflicts of interests (no material interest in any commercial entity with a product that could be affected by the guidelines) (see online Appendix for more information).
Formulation of Specific Clinical Questions and Determining Outcomes of Interest
The ONS Guidelines panel met biweekly to discuss and prioritize clinical questions for this guideline. Panelists were instructed to identify clinically relevant questions that patients were asking about lymphedema treatment and management, particularly questions whose answers posed uncertainty for clinicians. Questions were formulated using the PICO (Patient, Intervention, Comparator, and Outcome) components. The guidelines panel selected patient-important outcomes of interest for each question a priori. The panel discussed all possible outcomes, then prioritized outcomes based on their importance for patients and decision making (Guyatt, Oxman, Kunz, et al., 2011). The PICO questions are included in the online Appendix.
Synthesis of Evidence and Development of Recommendations
The evidence for this guideline was identified from two systematic reviews and meta-analyses of randomized controlled trials (RCTs) and nonrandomized studies on interventions for lymphedema conducted by researchers at the Mayo Clinic Evidence-Based Practice Center and McMaster University (Ding et al., 2020; Lytvyn et al., 2020).
The evidence from those reviews was assessed and summarized, using GRADE, and presented in an evidence profile. Within the evidence profile, the body of evidence across each outcome was assessed based on factors that either decreased or increased one’s certainty: risk of bias, inconsistency, indirectness, imprecision, publication bias, large magnitude of effect, dose-response gradient, or opposing residual confounding (Balshem et al., 2011; Guyatt, Oxman, Sultan, et al., 2011).
During a two-day in-person meeting, the panel developed clinical recommendations based on the evidence summarized in the evidence-to-decision (EtD) framework. For each recommendation, the panel decided on the following: the certainty in the evidence, the balance of benefits and harms of the compared intervention options, the patients’ values and preferences associated with the decision, resource use, health equity, acceptability of stakeholders, and feasibility. The panel also discussed the extent of the use of alternative treatment options. The panel agreed on the recommendations (including direction and strength), remarks, and qualifications by consensus vote based on the balance of all desirable and undesirable consequences. For each question, the panel entered judgments into the GRADE EtD framework using the GRADEpro Guideline Development Tool.
Interpretation of Recommendations
The recommendations in this guideline are labeled as strong, conditional, no recommendation, or research, according to the GRADE approach. Table 1 provides GRADE’s interpretation of the recommendations by patients, clinicians, healthcare policymakers, and researchers. The ONS recommendations for the management of lymphedema are summarized in Table 2. 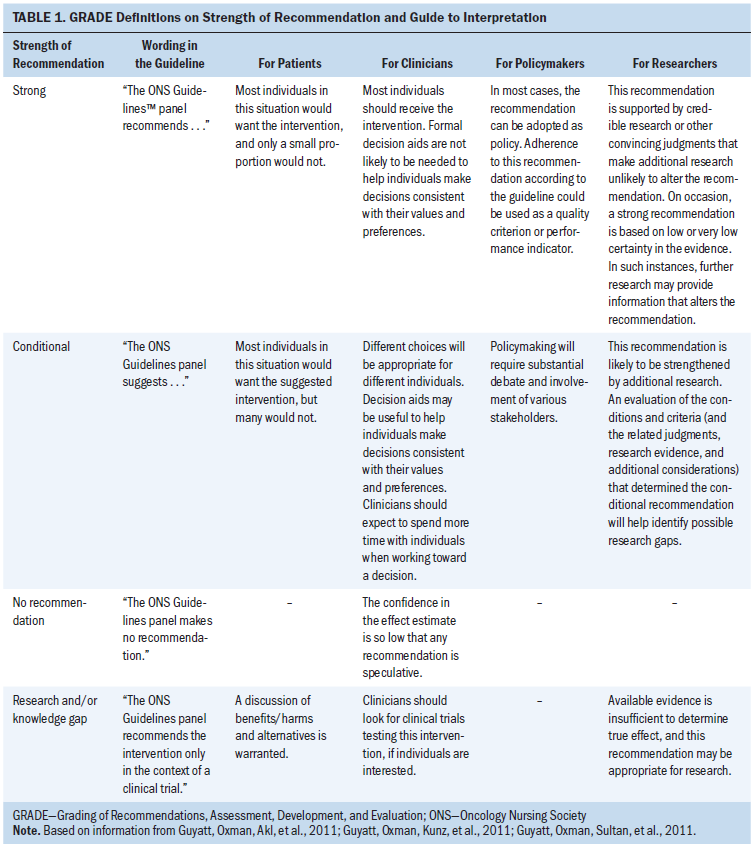
Document Review
Draft recommendations were reviewed and approved by all members of the guideline panel and then opened for public comment from January 10 to 24, 2020. Individuals or organizations submitted comments during the public comment period. In addition, a targeted peer review was conducted with three clinical research experts on lymphedema. The goal of the targeted peer review was to obtain direct feedback on the draft recommendations, as well as a professional consultation to facilitate dissemination of the final guideline to practitioners. The document was revised to address pertinent comments following public comment and targeted peer-review periods; however, no changes were made to the recommendations. The ONS Board reviewed and approved the guideline methodology and process. The guidelines were then submitted to the Oncology Nursing Forum for publication. 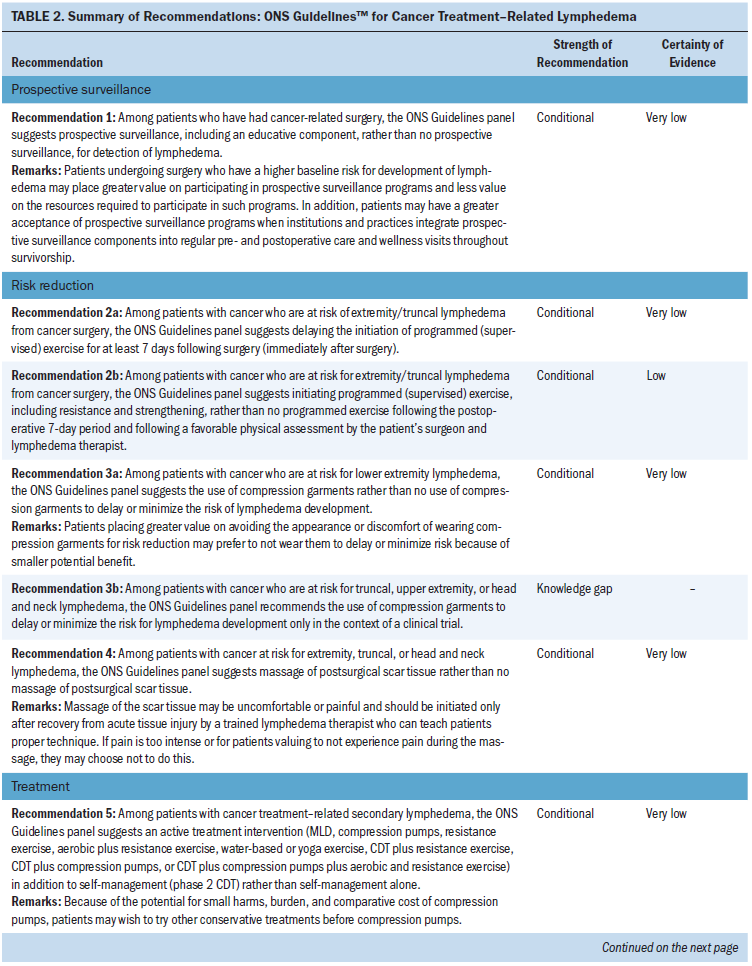
How to Use These Guidelines
The ONS Guidelines are intended to assist clinicians in making decisions about treatment interventions for common symptoms experienced by patients with cancer throughout the treatment trajectory. The ONS Guidelines are intended to inform education, identify research gaps, and promote policy and advocacy. They may also be used by patients in collaboration with their healthcare team. The ONS Guidelines are not medical advice and do not replace care by a cancer care clinician. Using a shared decision-making process, clinicians make decisions with patients, including discussion of patients’ values and preferences with respect to their current situation. The ONS Guidelines may not include all available treatments for an individual patient. Treatments described in the ONS Guidelines may not be appropriate for all patients or in all scenarios. As scientific advances and new evidence become available, the ONS Guidelines may become outdated. Following the ONS Guidelines does not guarantee improvement or a successful outcome. ONS does not warrant or guarantee any products described. Implementation of the ONS Guidelines will be facilitated by forthcoming interactive dissemination tools and patient education resources.
Recommendations, Key Evidence, and Qualifying Statements
The recommendations are organized in three main sections: prospective surveillance, risk reduction, and treatment for lymphedema. The prospective surveillance and risk-reduction recommendations are based on a systematic review and meta-analysis of 26 studies (19 RCTs) of 4,095 participants. Twenty-four publications (17 RCTs) are included in the quantitative synthesis (Ding et al., 2020). The treatment recommendations are based on a systematic review and network meta-analysis of 36 studies with 1,651 participants (Lytvyn et al., 2020). Treatment included all conservative treatment strategies of at least two weeks in duration, such as CDT, MLD alone, compression pumps, exercise (resistance, weight training, yoga, and water-based), and standard care (verbal instructions and printed educational information). Surgical treatments, pharmacologic treatments, laser therapy, kinesiotape, shock-wave therapy, electrical stimulation therapy, and aromatherapy were excluded. Also excluded were trials that compared different medical devices to each other (e.g., different brands of compression bandages, garments, or pumps) (Lytvyn et al., 2020).
The narrative following each recommendation parallels the organization of the GRADE EtD framework, with a summary of the evidence, followed by a description of the benefits and harms considered by the panel members, an inclusion statement about the certainty of the evidence, and a final summary of the recommendation, considering any overarching remarks made by the panel. Additional factors from the EtD framework are summarized in a later section in this article.
Prospective Surveillance
Recommendation 1
Among patients receiving cancer-related surgery, should prospective surveillance rather than no prospective surveillance be conducted to detect lymphedema?
Among patients who have had cancer-related surgery, the ONS Guidelines panel suggests prospective surveillance, including an educative component, rather than no prospective surveillance, for detection of lymphedema (conditional recommendation; very low certainty of evidence).
Remarks: Patients receiving surgery and who have a higher baseline risk for development of lymphedema may place greater value on participating in prospective surveillance programs and less value on the resources required to participate in such programs. Patients may have a greater acceptance of prospective surveillance programs when institutions and practices integrate prospective surveillance components into regular pre- and postoperative care and wellness visits throughout survivorship.
Summary of the Evidence
A systematic review identified two studies that addressed this question, with one reporting on a single-center surveillance program for lymphedema management (Yang et al., 2016) and another on a pre- and postoperative risk-reduction protocol (Boccardo et al., 2009). The surveillance program (Yang et al., 2016) included comprehensive surveillance by an interprofessional team with an emphasis on early detection and risk reduction of lymphedema. Patients who underwent axillary lymph node dissection and were considered at high risk of lymphedema were placed on a care plan immediately after surgery. The plan included a patient-reported symptom index and a multi-frequency bioelectrical impedance analyzer; however, the timing and frequency of these assessments were not clear. If subclinical lymphedema was diagnosed, compression interventions were started (compression sleeve fitted by a trained nurse). If volume increased (greater than 3%), the compression sleeve plus education on manual lymphatic massage were prescribed for four weeks. For recurring lymphedema at stage 0 to I, progressive weightlifting and strengthening exercises were started in a supervised program that continued at home (Yang et al., 2016). For clinical lymphedema at stage II or greater, CDT was prescribed as standard of care by specially trained lymphedema therapists.
Comparatively, a risk-reduction protocol from Boccardo et al. (2009) included preoperative upper limb lymphoscintigraphy, principles for lymphedema risk minimization, and early management of lymphedema when it was diagnosed. Patients were assessed preoperatively and 1, 3, 6, 12, and 24 months postoperatively.
Although education was not mentioned as a component in all the studies for this review, it is essential that a prospective lymphedema surveillance program include supportive educative resources as a key component. One of the main barriers to treatment and adherence to self-management of BCRL is lack of knowledge and support (Ostby & Armer, 2015). Education alone is inadequate. A supportive environment is also necessary to address psychological well-being and coping skills in maintaining adherence to BCRL self-management (Armer et al., 2011; Ostby et al., 2018).
Adherence to self-management is facilitated with an effective prospective surveillance program that contains physical assessment (early detection); symptom, functional, and quality-of-life assessment; and educational supportive materials for risk-reduction strategies.
Benefits
Prospective surveillance had a moderate effect on the likelihood of detection of lymphedema according to a single study (relative risk [RR] = 2.06, 95% confidence interval [CI] [1.54, 2.76]) (Yang et al., 2016). Preoperative surveillance also had a moderate effect on the development of lymphedema (RR = 0.24, 95% CI [0.06, 1.02]; absolute risk reduction [ARR] 253 fewer per 1,000, 95% CI [253 fewer, 7 more]) (Ding et al., 2020).
Harms and Burden
The prospective surveillance program did not report on adverse events related to the program components, but did note that poor compliance had a significant impact on lymphedema incidence (odds ratio [OR] = 2.98, p = 0.002) and that low level of self-monitoring and insight scores (one of the domains from the patient-reported health-related empowerment questionnaire) were significantly related to lymphedema incidence (OR = 1.31, p = 0.025), after adjusting for confounding variables such as age, body mass index, and type of cancer treatment (Yang et al., 2016). The pre- and postoperative protocol (Boccardo et al., 2009) also did not report on adverse events; however, the authors reported that two-year assessments were completed by 89% of the women in both study groups, signifying low attrition.
Certainty in the Evidence of Effects
The certainty in the evidence was rated as very low related to imprecision and risk of bias.
Other Evidence-to-Decision Criteria and Considerations
The ONS Guidelines panel judged the desirable effects to be large because they prioritized the outcome of diagnosis of early-stage lymphedema as an opportunity to identify lymphedema earlier and provide treatment or management at an earlier stage with optimal outcomes. The panel also noted that the cumulative incidence of advanced-stage (stage III) lymphedema was less in the surveillance group compared to historical controls (Yang et al., 2016). The panel judged the undesirable effects to be small, with potential patient burden that could be minimized with surveillance at the same time as regular checkups. The panel also did not expect harms from the components of the surveillance programs.
The ONS Guidelines panel noted possible important uncertainty or variability in how much people value the main outcome of lymphedema development—patients at higher risk might prefer to participate in a surveillance program, whereas patients at lower risk may prefer to not have the increased burden of additional visits and monitoring. The panel judged that the balance of effects favored the surveillance intervention for all risk groups. The resources required could be moderate, with costs for equipment, personnel, and data collection. Once a program is integrated into a health system, the costs would decrease. The panel judged that cost effectiveness favors surveillance, with the reported cost per year for a prospective surveillance model estimated at $636.19 and the cost to manage late-stage breast cancer–related lymphedema per year in a traditional model to be $3,124.92 (Stout et al., 2012, 2013). An economic analysis from Shih et al. (2009) found that women with BCRL had significantly higher overall medical costs compared to matched controls in the two years following treatment ($23,167 versus $14,877). Dean et al. (2019) found that monthly direct costs were 122% higher for breast cancer survivors with lymphedema compared to survivors without lymphedema; therefore, the long-term savings gained by risk reduction for the chronic sequelae of BCRL is warranted with prospective surveillance. The panel noted that there would likely be no impact on equity, and that surveillance is acceptable to key stakeholders and feasible to implement.
Conclusions
The ONS Guidelines panel determined that there is low certainty in the evidence for a net health benefit from surveillance, but that the desirable anticipated effects were large, and the balance of effect favors surveillance, rather than no surveillance. Based on this evidence, the panel issued a conditional recommendation in favor of prospective surveillance in patients at risk for cancer treatment–related lymphedema. Figure 1 includes considerations for components of a prospective surveillance program. Additional research is needed to inform specific components and identify outcomes from prospective surveillance programs. 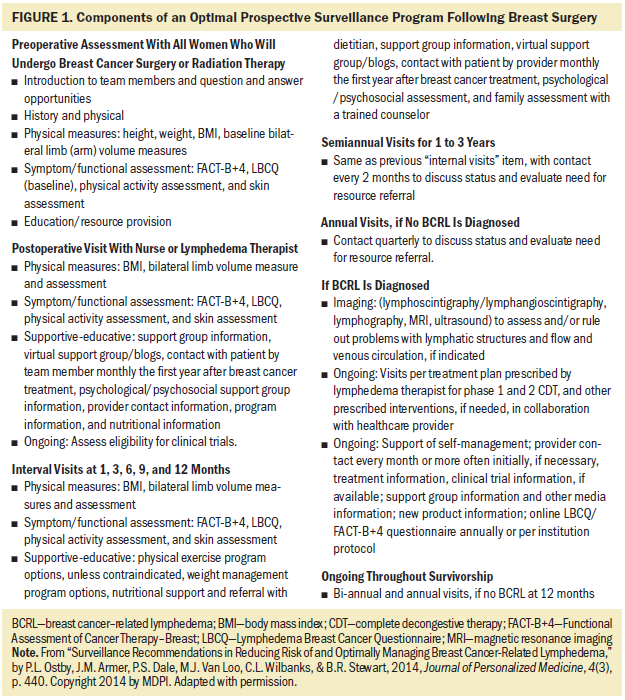
Risk Reduction
Recommendation 2
Among patients who are at risk for extremity or truncal lymphedema from cancer surgery, should programmed (supervised) exercise rather than no programmed exercise be initiated to delay or minimize the risk of lymphedema development?
Recommendation 2a: Among patients with cancer who are at risk of extremity/truncal lymphedema from cancer surgery, the ONS Guidelines panel suggests delaying the initiation of programmed (supervised) exercise for at least seven days following surgery (immediately postoperative) (conditional recommendation; very low certainty of evidence).
Recommendation 2b: Among patients with cancer who are at risk for extremity/truncal lymphedema from cancer surgery, the ONS Guidelines panel suggests initiating programmed (supervised) exercise, including resistance and strengthening exercises, rather than no programmed exercise following the postoperative seven-day period and following a favorable physical assessment by the patient’s surgeon and lymphedema therapist (conditional recommendation; low certainty of evidence).
Summary of the Evidence
The overarching question the panel included was focused on postoperative exercise, but the panel determined there to be two main subgroups—delayed exercise (more than seven days postoperative) compared to early (immediately postoperative) exercise and programmed exercise compared to no programmed exercise. The panel considered programmed exercise to be exercise, including arm/shoulder exercises, that was guided or supervised by a healthcare professional.
For the delayed exercise component of this question, the ONS Guidelines panel suggested delaying exercise at least seven days postoperatively compared with initiating exercise immediately following surgery. The systematic review identified two RCTs that addressed this question (Bendz & Fagevik Olsén, 2002; Todd et al., 2008). The study by Bendz and Fagevik Olsén (2002) included 230 women with breast cancer who were randomized to early (immediately postoperative) shoulder exercise or delayed shoulder exercise (starting at 14 days postoperative) and were followed for two years. The study by Todd et al. (2008) included 116 women with breast cancer who were randomized to delayed exercises compared to early full-range shoulder mobilization exercises following surgery with a follow-up of six months.
For programmed exercise compared to no exercise, the systematic review identified five RCTs (AmmitzbØll et al., 2019; Cinar et al., 2008; Kilbreath et al., 2013; Sagen et al., 2009; Schmitz et al., 2010) and two nonrandomized studies (Corrado et al., 2018; Sisman et al., 2012) that reported on the development of lymphedema as an outcome of interest. Sample sizes in the RCTs ranged from 57 to 204, with length of follow-up from six months to two years postintervention. The types of exercise varied, including resistance and strengthening exercises in a supervised and/or a home-based setting. Other forms of exercise (yoga, water-based) may be beneficial, and the panel considered these to have a resistance component that was similar to those in the reviewed studies. A common theme among all studies was that the exercise program was supervised for all or part of the study. All studies were with patients following breast cancer surgery.
Benefits
Delaying exercise (at least seven days postsurgery) had a moderate effect on reducing development of lymphedema (0.47; 95% CI [0.23, 0.96], ARR 70 fewer per 1,000, from 102 fewer to 5 fewer) and grip strength (RR = –0.03; 95% CI [–0.31, 0.26]).
Programmed exercise had a moderate effect on change in physical activity (RR = 3.54; 95% CI [2.67, 4.41]) and range of motion (shoulder flexion) (RR = 9.36; 95% CI [–2.36, 21.09]). The panel prioritized the outcomes of range of motion and change in physical activity when comparing programmed exercise to no programmed exercise because the eventual development of lymphedema may not change, but the well-being of the patients may improve.
Harms and Burdens
Exercise, whether early or delayed, did not appear to lead to significant harms for the patients. In the study by Bendz and Fagevik Olsén (2002), no patients graded any of their symptoms as severe. Todd et al. (2008) reported that postoperative wound drainage volumes were significantly greater in patients assigned to the early exercise group (p = 0.004). Adverse events were not reported in all studies that assessed programmed exercise. Sagen et al. (2009) reported that two participants developed frozen shoulder during the intervention program.
Certainty in the Evidence of Effects
For the question of delayed compared to early exercise, the certainty in the evidence was rated as very low because of imprecision from the potential of benefits and harms and for risk of bias.
For the question of programmed exercise compared to no exercise, the certainty of evidence across the body of evidence for the outcomes was low because of concerns with inconsistency between the findings from the RCTs and nonrandomized studies, as well as imprecision.
Other Evidence-to-Decision Criteria and Considerations
For the question of delayed compared to early exercise, the ONS Guidelines panel judged the desirable anticipated effects to be moderate, with an almost 50% reduction in the development of lymphedema. The panel judged the undesirable effects to be small, with improvements in grip strength and range of motion, but these improvements have uncertain clinical significance. The panel judged there to be no important uncertainty in how people would value the main outcome and that the balance of effects favors delayed exercise. The panel considered that there would be negligible resources required, as well as negligible costs and savings from delaying exercise for seven days. Cost effectiveness favors delayed exercise because of the increased long-term cost savings from reduced need for lymphedema treatment. The panel considered there to be no impact on equity with delayed exercise and that it would be acceptable to key stakeholders and feasible to implement.
For the question of programmed exercise compared to no exercise, the ONS Guidelines panel judged the desirable and undesirable effects to be small related to the uncertain clinical significance of a small change in outcome and the different programs and regimens included in the studies. The panel decided that there was possibly important variability in how much patients would be willing to commit to a programmed exercise program. This would depend on the variables of the exercise program (frequency, cost), as well as their considered risk of developing lymphedema. The panel judged that the balance of effects did not favor either programmed exercise or no exercise. The panel considered that the resources required would be moderate if the exercise program included the cost to a gym and a trainer with variable cost effectiveness. The panel considered that equity would be reduced with programmed exercise when considering accessibility, coverage, transportation to appropriate facilities, and trained providers. Key stakeholder acceptability would vary, although implementation of programmed exercise would be feasible. The panel noted that programmed exercise should be under the supervision of a lymphedema specialist and physical therapist with communication with the patients’ surgical team.
Conclusions
The ONS Guidelines panel determined there was very low certainty in the evidence for net health harms from delayed exercise compared to early postoperative exercise. Overall, the panel judged that the desirable outcomes were greater than the undesirable outcomes, and made a conditional recommendation for delayed exercise rather than immediate postoperative exercise.
Risk Reduction
Recommendation 3
Should patients undergoing cancer surgery use compression garments rather than no use of compression garments to delay or minimize the risk of lymphedema development?
Recommendation 3a: Among patients with cancer who are at risk for lower extremity lymphedema, the ONS Guidelines panel suggests use of compression garments rather than no use of compression garments to delay or minimize lymphedema development (conditional recommendation; very low certainty of evidence).
Remarks: Patients placing greater value on avoiding the appearance or discomfort of wearing compression garments may prefer to not wear them to delay or minimize lymphedema because of smaller potential benefit for risk reduction.
Recommendation 3b: Among patients with cancer who are at risk for truncal, upper extremity, or head and neck lymphedema, the ONS Guidelines panel recommends use of compression garments to delay or minimize lymphedema development only in the context of a clinical trial (knowledge gap; research recommendation).
Summary of the Evidence
The panel made the decision to divide this question into two categories, separating lower extremity from truncal, upper extremity, and head/neck because of the increased risks of lymphedema following inguinal lymph node dissection (Biglia et al., 2017). The panel acknowledged the very limited evidence for prophylactic use of lower extremity compression garments, and that this evidence is focused primarily on prevention of deep vein thrombosis and not lymphedema.
The systematic review identified two studies (Ochalek et al., 2019; Stuiver et al., 2013). During review, the panel removed the study by Ochalek et al. (2019) because it only studied compression garments during physical activity and was too indirect to inform this question. The study by Stuiver et al. (2013) included 80 patients with melanoma or urogenital cancers who underwent inguinal lymph node dissection. Patients were randomly assigned to compression stockings for six months or to a usual care group. Follow-up was assessed at 12 months.
The systematic review did not identify any additional studies of prophylactic garment use for patients with cancer at risk of truncal, upper extremity, or head and neck lymphedema.
Benefits
The primary outcome from the Stuiver et al. (2013) study was the first occurrence of lymphedema in the ipsilateral leg. At the six-month follow-up, 24 of 37 patients (65%) in the compression garment group and 26 of 32 patients (81%) in the control group had developed lymphedema (RR = 0.8, 95% CI [0.6, 1.07]). At 12-month follow-up, 28 of 36 patients (77%) in the compression garment group and 27 of 32 patients (84%) in the control group had developed lymphedema (RR = 0.92, 95% CI [0.73, 1.16]).
Harms and Burdens
No differences were seen between the compression garment group and the control group for wound complications, incidence of general edema or genital edema, body image, or quality of life. About one-third of patients reported that the lower extremity compression garment was uncomfortable (Stuiver et al., 2013).
Certainty in the Evidence of Effects
The ONS Guidelines panel rated the certainty in these estimated effects as very low owing to serious imprecision and risk of bias.
Other Evidence-to-Decision Criteria and Considerations
The ONS Guidelines panel rated the desirable and undesirable anticipated effects as small. The panel considered that the harms may be underreported and that patients may experience a skin allergy from the compression garments. The panel considered that there is possibly important uncertainty or variability in how much people value the main outcome of lymphedema. The panel considered the variability to be related to the aesthetic and comfort of wearing the garments weighed with a small risk of preventing an exacerbation of symptoms from compromised lymphatics. The panel judged the balance of effects to probably favor compression garments for lower extremities; however, the benefit for truncal, upper extremity, or head and neck prophylaxis is unknown.
Moderate costs are required because custom garments may be needed, including replacement about every six months. Based on the results of the studies reviewed, there is reason to believe that positive outcomes with slowed lymphedema progression can be quantified as cost savings compared to care costs for advanced-stage lymphedema and subsequent complications. Health equity would probably be reduced for patients without insurance coverage for the garments and accessibility to custom garments.
Conclusions
The ONS Guidelines panel determined there was very low certainty in the evidence for net health harms from compression garments for risk reduction of lower extremity lymphedema. Overall, the panel judged that the desirable outcomes, although minimal, were greater than undesirable outcomes and made a conditional recommendation for compression garments for reducing risk of lower extremity lymphedema. The panel considered the limited evidence on prophylactic use of lower extremity compression garments with the risk of lymphedema following inguinal lymph node dissection and acknowledged that this is a situation where shared decision making between the patient and the healthcare provider should occur in weighing individual risks and harms.
The panel decided that the evidence for the benefits was too indirect to inform a recommendation for patients at risk of truncal, upper extremity, or head and neck cancer; however, the harms associated with the compression garment may be similar. Therefore, the panel decided to make a research recommendation that compression garments to delay or minimize the risk of lymphedema in patients with truncal, upper extremity, or head and neck cancer be used only in the context of a clinical trial.
Risk Reduction
Recommendation 4
Among patients with cancer at risk of developing lymphedema, should massage of scar tissue rather than no massage of scar tissue be performed?
Among patients with cancer at risk for extremity, truncal, or head and neck lymphedema, the ONS Guidelines panel suggests massage of postsurgical scar tissue rather than no massage of postsurgical scar tissue (conditional recommendation; very low certainty of evidence).
Remarks: Massage of the scar tissue may be uncomfortable or painful and should be initiated only after recovery from acute tissue injury by a trained lymphedema therapist who can teach patients proper technique. If pain is too intense or for patients valuing to not experience pain during the massage, they may choose not to do this.
Summary of the Evidence
The panel identified a systematic review that addressed this question in a non–cancer-related lymphedema population (Shin & Bordeaux, 2012). This review included 10 publications, summarizing data from 144 patients who received scar massage following surgery or burns. In the Shin and Bordeaux (2012) review, time to massage ranged from suture removal to more than two years after surgery. Massage protocols ranged from 10 minutes twice daily to 30 minutes twice weekly. Treatment duration for the studies varied from a one-time massage to massages over a six-month time frame (Shin & Bordeaux, 2012). The panel’s review identified one trial examining prophylactic scar massage among patients with breast cancer in ClinicalTrials.gov; however, no results could be located and the study was not completed. The panel acknowledged that scar tissue may have different clinical presentations and intervention strategies (Leask et al., 2002; Stubblefield, 2011), which underscores the importance of management from a certified therapist with experience in lymphedema.
Massage of scar tissue is taught by a certified lymphedema therapist and can then be self-administered by the patient following the therapist’s instruction. Ongoing monitoring by a lymphedema therapist is important to verify patient technique and maintain consistency. The panel noted that massage of scar tissue is distinct from MLD.
Benefits
Overall, in Shin and Bordeaux’s (2012) review, 65 of 144 (46%) patients with scars or burns (not related to lymphedema or cancer treatment) saw an improvement (based on objective and subjective outcomes) and 90% (n = 27 of 30) experienced improved appearance or an improved scar assessment scale score (Shin & Bordeaux, 2012).
Harms and Burdens
The studies included in this review varied regarding when treatment should be initiated, massage protocol and duration, outcomes evaluated, and how the outcomes were measured. Potential harms include conducting an ineffective treatment, irritation from friction, and possible skin rash or irritation from the lubricant used for massage (Shin & Bordeaux, 2012).
Certainty in the Evidence of Effects
The panel determined the certainty of evidence to be very low for this question because of the indirectness of the evidence, which was not specifically from patients undergoing surgery for cancer or in radiation therapy–treated tissue, as well as the lack of evidence around the potential harms from massage of scar.
Other Evidence-to-Decision Criteria and Considerations
The ONS Guidelines panel judged the desirable effects to be large because scars may limit range of motion, which is important for daily functioning. Appearance may also be an issue for patients, and both can be surrogates for quality of life and self-image. The undesirable effects were judged to be small, with the possibility of temporary pain during the massage. The panel judged there to be probably no important uncertainty or variability in how much people valued the main outcomes, and that the balance of effects favors prophylactic massage of the scar. The panel considered that the harms may be underreported in the literature, but they are expected to be minimal and resolve quickly, with a large expected benefit.
The panel considered the resources required to be moderate, including an office visit for teaching, but the cost effectiveness probably favors the intervention, although there were no identified cost-effectiveness studies. The panel determined that there would be an increase in equity because patients can learn to do this at home—the only cost would be an office visit. However, the office visit may not be covered (reducing equity), especially if services were provided by a specialist. Access to a properly trained specialist could be a source for reduced equity. The panel judged that massage was acceptable to key stakeholders and was feasible to implement.
The panel noted that within cancer treatment–related lymphedema risk reduction, self-directed MLD and guided progressive exercise are important to reducing the progression of scar adhesion. In general, nurses can help with scar management by providing basic active range of motion exercises within safe progression. Massage techniques over a surgical scar traditionally are not started until the end of the fibroplasia stage of wound healing (four to six weeks after surgery). The panel emphasized that to maximize the effect of scar massage, adequate technique and patient’s compliance to scar massage are critical. Therapists should evaluate if the patient replicates the scar massage technique correctly and if the patient adheres to the prescribed regimen for scar management.
Conclusions
To date, there is a lack of evidence regarding the effect of massage of scar in radiation therapy–treated tissue. Therefore, this recommendation is only applicable to postsurgical scar management. The ONS Guidelines panel determined that there is very low certainty in the evidence for a net health benefit from massage, but that the desirable anticipated effects were large, and the balance of effect favors massage, rather than no treatment. Based on this evidence, the panel issued a conditional recommendation in favor of scar massage in patients at risk for cancer treatment–related lymphedema.
Treatment
Current standard of care for lymphedema treatment is CDT, including intensive lymphedema therapy (phase 1 of CDT) with a certified lymphedema therapist, followed by long-term self-management (phase 2 of CDT) administered by the patient and/or a caregiver. The intensive phase includes MLD, application of compression bandages (23 of 24 hours for 7 days per week), exercise, and meticulous skin and nail care. The self-management phase 2 of CDT includes self-administered MLD, application of compression garments and/or bandages, exercise, and continued meticulous skin and nail care. Follow-up should include monitoring of self-care practices with periodic assessment of soft tissue changes, which would require alterations in the self-care plan (Deng et al., 2019; Lasinski et al., 2012). The following questions refer to phase 2 of CDT as patient self-management and specifically addresses the addition of interventions to phase 2 of CDT.
Recommendation 5
Among patients with cancer treatment–related secondary lymphedema, should any additional active treatment be used with self-management (phase 2 CDT) for treatment of lymphedema?
Among patients with cancer treatment–related secondary lymphedema, the ONS Guidelines panel suggests active treatment in addition to self-management (phase 2 CDT) rather than self-management alone (conditional recommendation; very low certainty of evidence). Interventions reviewed include MLD, compression pumps, resistance exercise, aerobic plus resistance exercise, water-based or yoga exercise, CDT plus resistance exercise, CDT plus compression pumps, or CDT plus compression pumps plus aerobic and resistance exercise.
Remarks: Because of potential small harms, burden, and comparative cost of compression pumps, patients may wish to try other conservative treatments before compression pumps.
Summary of the Evidence
The systematic review identified 36 RCTs with 1,561 patients that addressed the question (Lytvyn et al., 2020). Interventions included MLD, aerobic and resistance exercise, compression pumps, water-based (aqua lymphatic) exercise, yoga and tai chi–like exercise, and self-management, which was defined as the use of self-massage, compression bandages, and/or garments; remedial exercise (e.g., active, repetitive, nonresistive motion); and skin and nail care (phase 2 of CDT). Excluding postsurgical swelling, studies included BCRL incidence as soon as three months post–cancer treatment. Length of time for the interventions studied varied from 2 to 52 weeks, with sample sizes ranging from 11 to 139 participants.
Benefits
Of the included studies, 35 reported on lymphedema volume. Of these, 27 were included in the network meta-analysis, and eight were reported narratively (N = 1,330 participants). Based on low to very low certainty of evidence, in a comparison of all interventions to self-management alone, there was no meaningful change in lymph volumes (Lytvyn et al., 2020). There was low to very low certainty of evidence of meaningful change in lymphedema volume when comparing conservative (nonsurgical) lymphedema treatments. The evidence suggests that MLD, compression pumps, resistance exercise, CDT, and aerobic plus resistance exercise may result in little to no difference in lymphedema volume changes when compared with self-management alone (standard mean difference [SMD] = –0.33, 95% CI [–1.07, 0.41]; SMD = –0.08, 95% CI [–0.82, 0.66]; SMD = 0.01, 95% CI [–0.48, 0.5]; SMD = 0.07, 95% CI [–0.29, 0.43]; and SMD = 0.19, 95% CI [–0.34, 0.72], respectively). In addition, water-based or yoga exercise, CDT plus resistance exercise, CDT plus compression pumps, and CDT plus compression pumps plus aerobic and resistance exercise have little to no effect on lymphedema volume, but the evidence is uncertain (SMD = –0.27, 95% CI [–0.74, 0.19]; SMD = –0.26, 95% CI [–0.98, 0.47]; SMD = –0.23, 95% CI [–0.83, 0.36]; and SMD = –0.12, 95% CI [–1.2, 0.96], respectively).
There was very low certainty of evidence of a small benefit for aerobic and resistance exercise compared to self-management for lymphedema swelling and symptoms (SMD = –0.38, 95% CI [–0.72, –0.05]), function (SMD = 1.87, 95% CI [1.27, 2.46]), and pain (SMD = –2.02, 95% CI [–2.63, –1.41]), based on one study with 63 participants (Park, 2017). There was very low certainty of evidence of a small benefit for lymphedema swelling and symptoms for the self-management group, compared to the CDT and compression pumps group (SMD –0.4, 95% CI [–0.73, –0.06]), based on two studies with 139 participants (Haghighat et al., 2010; Szolnoky et al., 2009). There was no statistically significant effect for any other comparisons or interventions, which were based on very low certainty of evidence.
Harms and Burdens
Among the included studies, nine reported on adverse events. Of these, four studies reported no adverse events and five studies reported adverse events, including withdrawals by participants potentially related to adverse events of temporary rash, pain in the affected arm, skin reaction to bandaging, discomfort from bandaging, lymphedema exacerbations, and infection/cellulitis.
Certainty in the Evidence of Effects
The panel rated the certainty in these estimated effects as very low owing to serious imprecision from the potential for both benefit and harm and risk of bias. The panel had concerns with the studies included in the network meta-analysis because many did not provide standard intervention components and had considerable variability in the baseline lymphedema volume/stage among participants within the same study. The panel also noted some issues with trial design, including lack of blinding of patients, influencing reporting of subjective outcomes, and lack of independent outcome assessment, as well as small sample sizes and participant withdrawals leading to incomplete outcome data.
Other Evidence-to-Decision Criteria and Considerations
The ONS Guidelines panel judged there to be trivial desirable effects after considering the variability in the underlying patient populations and the stage of lymphedema in the reported studies. Self-management varied across the comparisons, which may affect the comparative efficacy of the studies. In the included studies, self-management included varieties of compression garments, simple lymphatic drainage, remedial exercise, and skin and nail care.
The panel judged that the undesirable effects vary with the intervention. Any intervention may cause some amount of discomfort, with compression pumps having a small risk of harm (discomfort or skin irritation) (Feldman et al., 2012). The interventions may also lead to patient burden in requiring extra visits to a healthcare provider and time to learn proper technique in using the intervention. The panel judged that there is probably no important uncertainty or variability in the main outcomes because the relief of symptoms is important to the patients. The panel considered that the balance between desirable and undesirable effects does not favor either the interventions or the comparison. The panel also noted that the desirable and undesirable effects may have an impact on adherence and subsequent effect on successful management. Resources required can vary across the different interventions. Compression pumps may be expensive, but also may be reimbursed by insurance. Compression garments or bandages do have an associated cost, but it is less than compression pumps. Aerobic and resistance exercise may have negligible costs, and MLD as part of the self-management component of phase 2 CDT may lead to moderate savings. No studies of cost effectiveness were identified. Health equity and disparities would vary by community, individual, health insurance coverage, and accessibility. The panel judged the interventions to be acceptable to stakeholders, except for compression pumps as a sole treatment; the panel acknowledged that compression pumps would be used as an adjunct with another treatment. The panel judged that the interventions would be feasible to implement, although feasibility can be a challenge when components of the interventions are not standardized. The panel also noted the importance of the exercise being supervised by someone with experience in exercise for cancer survivors to monitor technique and support continuity of the exercise program. The panel also noted the benefits of exercise in general for cancer survivors and that these exercises have not been shown to cause or exacerbate lymphedema.
Conclusions
The ONS Guidelines panel determined there was very low certainty in the evidence for net health harms from MLD, aerobic and resistance exercise, compression pumps, water-based (aqua lymphatic) exercise, and yoga and tai chi–like exercise, in addition to self-management. Overall, the panel judged that the desirable outcomes outweighed the undesirable outcomes and made a conditional recommendation for any one or a combination of the interventions listed in addition to self-management.
Treatment
Recommendation 6
Among patients with cancer treatment–related secondary lymphedema, should resistance exercise plus self-management (phase 2 CDT) rather than self-management alone be used for lymphedema treatment?
Among patients with cancer treatment–related secondary lymphedema, the ONS Guidelines panel suggests resistance exercises in addition to self-management (phase 2 CDT) rather than self-management alone (conditional recommendation; very low certainty of evidence).
Remarks: Preference for resistance exercises may be driven by cost and accessibility.
Summary of the Evidence
The systematic review identified three RCTs that addressed this question (Cormie et al., 2013; Jeffs & Wiseman, 2013; Schmitz et al., 2009). Two of the resistance exercise programs were partially gym-based and partially home-based (Cormie et al., 2013; Schmitz et al., 2009), and one was home-based (Jeffs & Wiseman, 2013). Sample sizes ranged from 23 to 139 participants.
Benefits
Resistance exercise had no significant effect on lymphedema volume (SMD = 0.01, 95% CI [–0.48, 0.5]) compared to standard care, based on low certainty of evidence. There was very low certainty of evidence that resistance exercise improves pain (SMD = –1, 95% CI [–1.57, –0.43]) and function measures (SMD = 2.49; 95% CI [1.79, 3.19]) based on one trial with 62 participants (Cormie et al., 2013). There was also very low certainty of evidence of a reduction in lymphedema swelling and symptoms (SMD = –0.38, 95% CI [–0.72, -0.05]) based on one trial with 139 participants (Schmitz et al., 2009). There were no statistically significant differences in any of the other outcomes.
Harms and Burdens
Overall, resistance exercise was well tolerated and acceptable to patients. No lymphedema exacerbations or other adverse events were reported in the studies (Cormie et al., 2013; Jeffs & Wiseman, 2013; Schmitz et al., 2009).
Certainty in the Evidence of Effects
The panel rated the certainty in these estimated effects as very low owing to very serious imprecision from the potential for both benefit and harm, and few participants included in the studies.
Other Evidence-to-Decision Criteria and Considerations
The panel judged the desirable effects to be small, including a decrease in lymphatic swelling and symptoms, an improvement in function, and a decrease in pain. The panel judged the undesirable effects to be small, with the potential burden of travel to and from training locations and with a small physical risk of injury or muscle strain with resistance exercise. The panel considered there to be no important uncertainty or variability in how much people value the main outcomes and that the balance of effects did not favor either resistance exercise or self-management. The resources required would result in negligible costs and savings, with no studies on cost effectiveness identified. The panel considered resistance exercises to be acceptable and feasible to key stakeholders. The panel noted that guidance from a trained professional is important to teach patients proper techniques and advise on gradual increase in resistance as well as reinforce continuity of an exercise program. The panel also noted the benefits of exercise in general for cancer survivors and that these exercises have not been shown to cause or exacerbate lymphedema.
Conclusions
The ONS Guidelines panel determined there was very low certainty in the evidence for net health harms from resistance exercises in addition to self-management. The panel noted the importance of the inclusion of a trained professional to supervise the exercise program. Overall, the panel judged that the desirable outcomes were greater than undesirable outcomes and made a conditional recommendation for resistance exercise in addition to self-management.
Treatment
Recommendation 7
Among patients with cancer treatment–related secondary lymphedema, should supervised water-based or yoga exercise plus self-management (phase 2 CDT), rather than self-management alone, be used for lymphedema treatment?
Among patients with cancer treatment–related secondary lymphedema, the ONS Guidelines panel suggests supervised water-based activities or yoga in addition to self-management (phase 2 CDT) rather than self-management alone (conditional recommendation; very low certainty of evidence).
Remarks: Preference for water-based exercise or yoga or self-management may be driven by cost and accessibility.
Summary of the Evidence
The systematic review identified five RCTs (Johansson et al., 2013; Letellier et al., 2014; Loudon et al., 2014; McClure et al., 2010; Pasyar et al., 2019) that addressed this question. Two focused on water-based therapy (Johansson et al., 2013; Letellier et al., 2014) and three on yoga or movement-based therapy (Loudon et al., 2014; McClure et al., 2010, Pasyar et al., 2019). All interventions included a supervised component, with some also including a home-based component as well. The water-based and yoga interventions were described as low to moderate intensity, and some included relaxation and meditation components. Length of intervention ranged from two to five months, with weekly or biweekly sessions. Sample sizes for treatment groups ranged from 18 to 23 participants.
Benefits
Water-based exercise or yoga had no statistically significant effect on lymphedema volume (SMD = –0.27, 95% CI [–0.74, 0.19]) based on very low certainty of evidence. There was very low certainty of evidence that water-based exercise or yoga improves pain compared to standard of care (SMD = –0.6, 95% CI [–1.05, –0.16]), based on three studies with 81 participants (Letellier et al., 2014; Loudon et al., 2014; McClure et al., 2010; Pasyar et al., 2019).
Harms and Burdens
Overall, water-based and yoga exercise was well tolerated and acceptable to patients. No lymphedema exacerbations or other adverse events were reported in the studies (Johansson et al., 2013; Letellier et al., 2014; Loudon et al., 2014; McClure et al., 2010).
Certainty in the Evidence of Effects
The ONS Guidelines panel rated the certainty in these estimated effects as very low, owing to very serious imprecision from the potential for both benefit and harm, few participants included in the studies, and serious risk of bias from high loss to follow-up.
Other Evidence-to-Decision Criteria and Considerations
The panel judged the desirable effects to be small in favor of water-based exercise or yoga for the outcomes of swelling or symptoms, pain, fatigue, and function, and the undesirable effects of water-based exercise or yoga to be trivial. The panel considered that there was no important uncertainty or variability in how much people value the main outcomes in that patients may prefer different interventions or self-management alone. The panel considered the balance of effects to neither favor water-based exercise nor yoga, as there were negligible differences between the two. The panel judged resources required to be moderate and did not identify cost-effectiveness studies. Equity would likely be reduced because there may be a cost for access to a pool or gym facility that would not be covered by insurance, as well as possible transportation costs. The panel considered that water-based exercise or yoga would be acceptable and feasible for key stakeholders. The panel also noted the importance of the exercise being supervised by someone with experience in exercise for cancer survivors to monitor technique and support continuity of an exercise program. The panel also noted the benefits of exercise in general for cancer survivors and that these exercises have not been shown to cause or exacerbate lymphedema.
Conclusions
The ONS Guidelines panel determined there was very low certainty in the evidence for net health harms from supervised water-based exercises or yoga in addition to self-management. Overall, the panel judged that the desirable outcomes were greater than the undesirable outcomes and made a conditional recommendation for either supervised water-based exercise or yoga, in addition to self-management.
Discussion
Other Guidelines on Lymphedema
The members of the ONS Guidelines panel were not able to identify a dedicated guideline for cancer treatment–related lymphedema in the literature; however, recommendations are included within other guidelines (e.g., breast cancer or survivorship, general lymphedema) and in position or consensus documents that provide guidance for lymphedema. Each guideline had limitations according to the AGREE II criteria (O’Donnell et al., 2020).
The International Society of Lymphology (ISL, 2020) has published a consensus document on diagnosis and treatment of peripheral lymphedema. The International Lymphedema Framework (ILF) has two consensus documents on best practice for the management of lymphedema (ILF, 2006) and compression therapy (ILF, 2012). Several national organizations (ACS/American Society of Clinical Oncology [ASCO], National Comprehensive Cancer Network [NCCN], and Society for Integrative Oncology) include guidance on lymphedema within guidelines for breast cancer or survivorship (Greenlee et al., 2017; NCCN, 2019; Runowicz et al., 2016). Each was developed with varying methodologies and timeframes for evidence review, but, when analyzed, the guidelines offer insight into consistency of recommendations and identify gaps in knowledge.
A consistent theme about these guidelines and the current guideline is the importance of education and involvement of a therapist knowledgeable about the diagnosis and treatment of lymphedema (trained and certified lymphedema therapist). Education is an important component of self-care and should be included throughout the lymphedema surveillance and treatment trajectory. Involving a lymphedema therapist is important for monitoring, early diagnosis, and treatment, such as fitting of compression garments, performance of CDT, and supervising exercise training (NCCN, 2019). The ACS/ASCO guideline on breast cancer survivorship includes a recommendation that primary care providers should counsel survivors on risk reduction and refer patients to a therapist knowledgeable about lymphedema, if needed (Runowicz et al., 2016).
Prospective surveillance is recommended by NCCN (2019) and ISL (2020), consistent with the current guideline. The NCCN specifically recommends pretreatment limb measurements as a baseline and notes the importance of treatment-related or individual risk factors guiding follow-up (NCCN, 2019). ISL (2020) notes the importance of identifying subclinical lymphedema on reducing the likelihood of progression to a chronic, advanced stage. For treatment, the Society for Integrative Oncology recommends low-level laser therapy (grade C level of evidence), which the ONS Guidelines panel did not include in the PICO questions. ISL recommends CDT for treatment of lymphedema, consistent with ONS Guidelines where the panel considered CDT to be standard of care. Compression garments for the treatment of lymphedema are recommended by NCCN and ISL, with the ISL noting that they should be prescribed by a clinician with expertise in lymphedema because some patients may have contraindications to compression garments. Resistance exercise is recommended by NCCN, under the supervision of a specialist, which is consistent with the ONS Guidelines.
Clinical Implications
The recommendations in this clinical practice guideline are grouped into three main categories: prospective surveillance, risk reduction, and conservative (nonsurgical) treatment. The ONS Guidelines panel recommended prospective surveillance and, for risk reduction, the panel recommended scar massage and delaying initiation of exercise until seven days after surgery. Compression garments for risk reduction are only recommended for patients at risk for lower extremity lymphedema. CDT is considered by the ONS Guidelines panel to be standard of care. The panel recommends the addition of resistance exercise, water-based exercise, or yoga to CDT for treatment of lymphedema. It is important to note that programmed exercise should be supervised by a professional with experience in treating patients with lymphedema to ensure proper technique as well as consistency and sustainability of the exercise program. The body of evidence evaluated for this guideline included different types of exercise and different regimens, highlighting the need for individualized exercise programs, particularly in light of the importance of exercise to cancer survivors in general (Cavanaugh, 2011; McTiernan et al., 2019). 
The ONS Guidelines panel recommendations were made based on currently available evidence. Additional research is needed to further define effective prospective surveillance, risk reduction, and treatment of lymphedema (see Figure 2). It will be important to standardize outcomes from these studies to include patient-reported outcomes and standard endpoints to enable evidence to be interpreted across studies and incorporated into patient care.
Conclusion
Patients have a lifelong risk of developing lymphedema after surgical and radiation therapy treatment for cancer. Lymphedema has the potential to be a debilitating and long-term/late side effect, if not diagnosed early and appropriately treated. Evidence-based guidance for clinicians on the surveillance, risk reduction, and treatment of lymphedema has the potential to improve patient care and outcomes.
The authors gratefully acknowledge Julia R. Rodrick, OTR/L, CLT-LANA®, WCC, and Nancy Hutchinson, MD, CLT-LANA®, for their thoughtful review of a draft of the recommendations.
About the Author(s)
Jane M. Armer, PhD, RN, FAAN, CLT, is a professor emerita and Pamela L. Ostby, PhD, RN, OCN®, CLT, is a research collaborator, both in the Sinclair School of Nursing at the University of Missouri in Columbia; Pamela K. Ginex, EdD, RN, OCN®, is the senior manager of evidence-based practice and inquiry at the Oncology Nursing Society in Pittsburgh, PA; Marcia Beck, RN, MSN, ACNS-BC, CLT-LANA®, is a lymphedema nurse coordinator in the University of Kansas Health System in Kansas City, KS; Jie Deng, PhD, RN, OCN®, FAAN, is an associate professor in the School of Nursing at the University of Pennsylvania in Philadelphia; Mei R. Fu, PhD, RN, FAAN, is the Barry Family and Goldman Sachs Endowed professor in the William F. Connell School of Nursing at Boston College in Massachusetts; Bonnie B. Lasinski, MA, PT, CLT-LANA®, is retired and residing in Pawleys Island, SC; Suzy Lockwood, PhD, MSN, RN, OCN®, FAAN, is an associate dean for nursing and nurse anesthesia and the director of the Center for Oncology Education and Research in the Harris College of Nursing and Health Sciences at Texas Christian University in Fort Worth; Ellen Poage, FNP-C, MSN, MPH, CLT-LANA®, is a lymphedema therapist nurse practitioner at Blackwell Breast Surgery, 21st Century Oncology Inc., in Fort Myers, FL; Joan White is the director at Lighthouse Lymphedema Network of Greater Atlanta in Georgia; Christine Maloney, BA, was, at the time of the writing, an archivist, Kerri A. Moriarty, MLS, is a research specialist, and Mark Vrabel, MLS, AHIP, ELS, was, at the time of the writing, an information resources supervisor, all at the Oncology Nursing Society in Pittsburgh, PA; and Rebecca L. Morgan, PhD, MPH, is an assistant professor in the Department of Health Research Methods, Evidence, and Impact, at McMaster University in Hamilton, Ontario, Canada. Armer, Ginex, Ostby, Deng, Fu, Lasinski, Lockwood, Poage, and Morgan contributed to the conceptualization and design. Armer, Ginex, Ostby, Deng, Fu, Lockwood, Maloney, Moriarty, and Morgan contributed to the data collection. Ginex, Ostby, Deng, and Morgan provided statistical support. Armer, Ginex, Ostby, Beck, Deng, Fu, Lockwood, White, and Morgan provided the analysis. Armer, Ginex, Ostby, Beck, Deng, Fu, Lasinski, Lockwood, Vrabel, and Morgan contributed to the manuscript preparation. Development of this guideline was wholly funded by the Oncology Nursing Society (ONS), a nonprofit nursing specialty organization that represents oncology nurses. ONS staff supported panel appointment and coordinated meetings but had no role in guideline question identification or voting on recommendations. Members of the guideline panel received travel reimbursement to attend one in-person meeting at ONS headquarters in Pittsburgh, PA. No honoraria were provided. Armer can be reached at armer@missouri.edu, with copy to ONFEditor@ons.org. (Submitted May 2020. Accepted May 8, 2020.)




